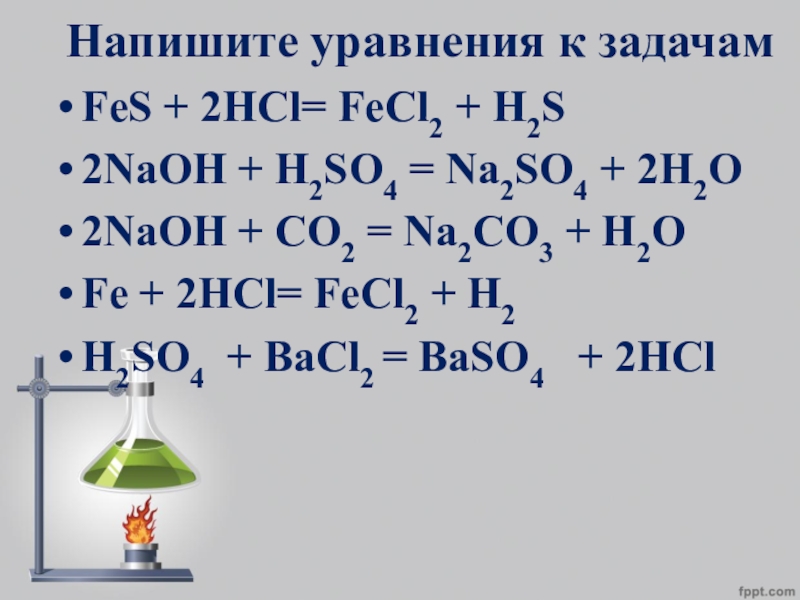
H2S является окислителем, Fe является восстановителем. Реактанты: Fe. Названия: Железо , Fe , Феррум. H2S – Сульфид водорода. Другие названия: Сероводород , …
H2S является окислителем, Fe является восстановителем. Реактанты: Fe. Названия: Железо , Fe , Феррум. H2S – Сульфид водорода. Другие названия: Сероводород , …
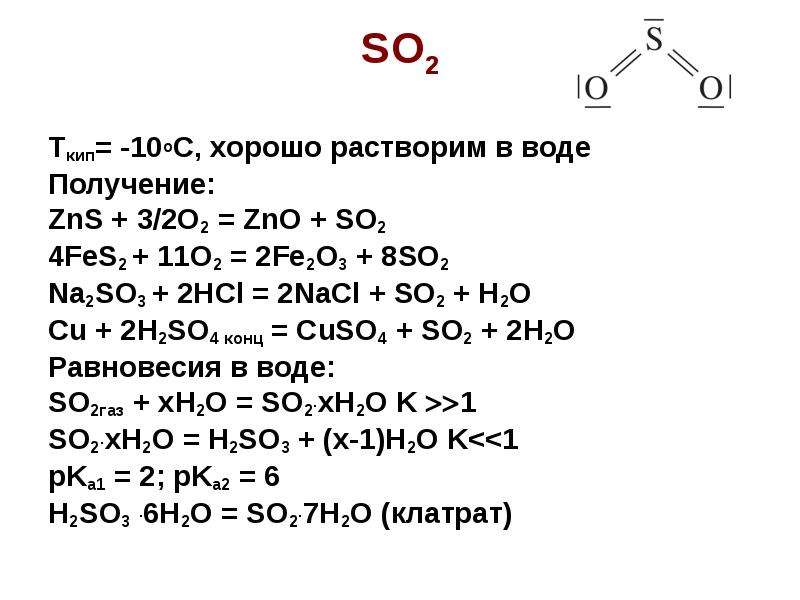
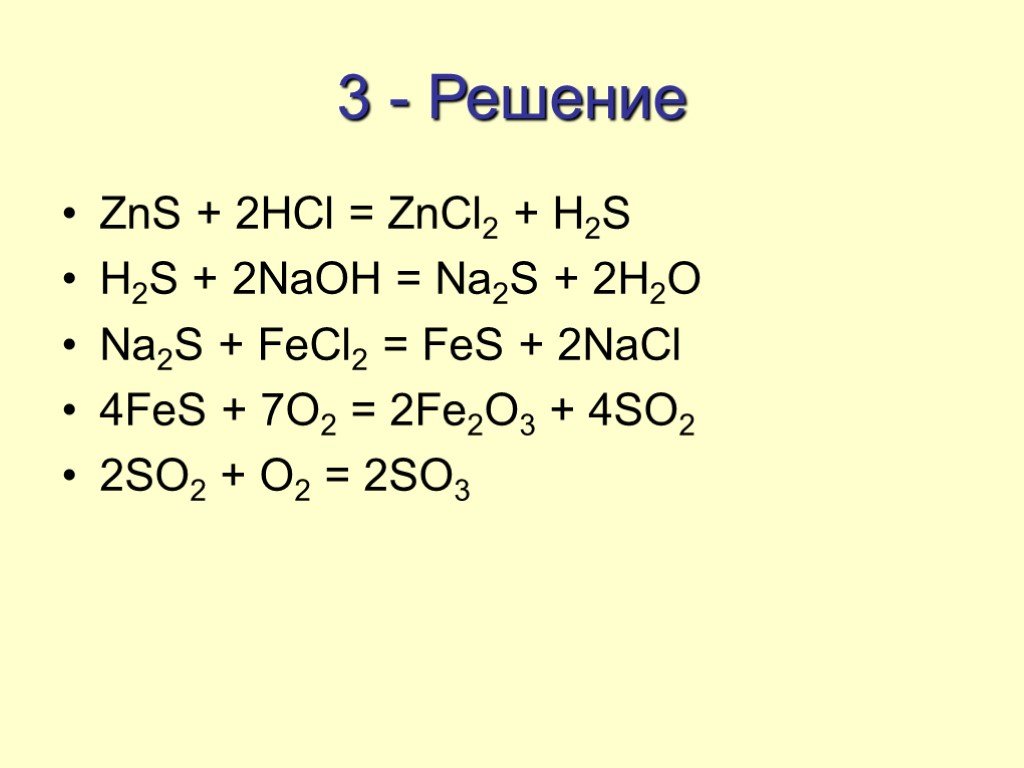
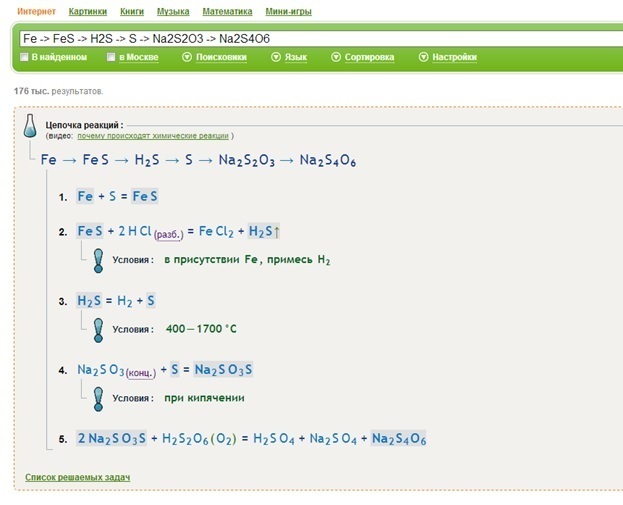 Луч Надежды Высший разум (108823) 13 лет назад. S → FeS → H2S → SO2 → Na2SO3 → Na2SO4 → BaSO4. Fe + S = FeS. FeS + 2HCl (разб. ) = FeCl2 + H2S↑. O3 + H2S (газ) = …
Луч Надежды Высший разум (108823) 13 лет назад. S → FeS → H2S → SO2 → Na2SO3 → Na2SO4 → BaSO4. Fe + S = FeS. FeS + 2HCl (разб. ) = FeCl2 + H2S↑. O3 + H2S (газ) = …
 With an annual formation of at least 5 million tons, pyrite (FeS 2) is the thermodynamically stable end product of iron compounds reacting with sulfide in reduced sediments, with the latter being produced mainly by microbial …
With an annual formation of at least 5 million tons, pyrite (FeS 2) is the thermodynamically stable end product of iron compounds reacting with sulfide in reduced sediments, with the latter being produced mainly by microbial …
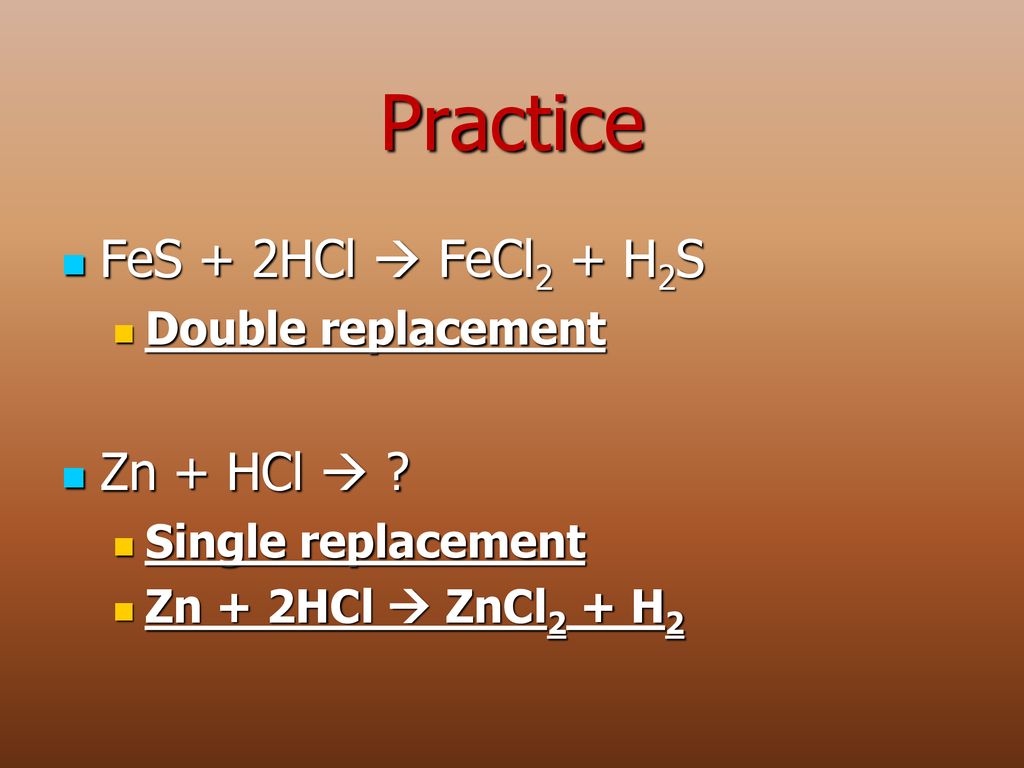
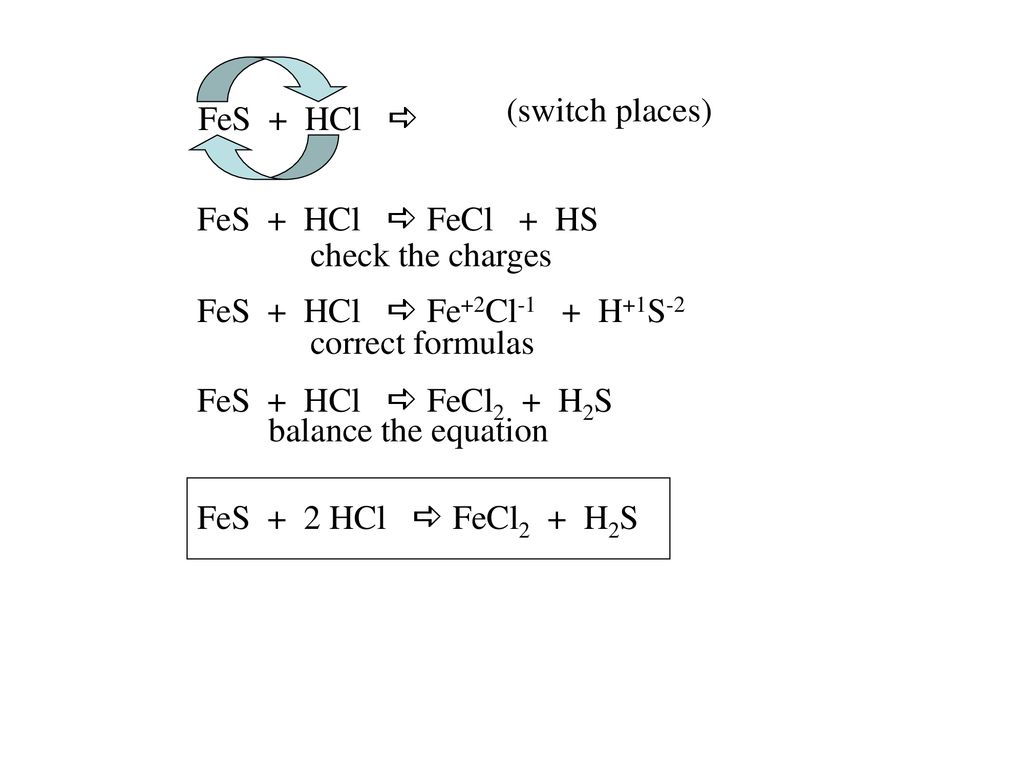
 Решенное и коэффициентами уравнение реакции FeS + 2 HCl → FeCl2 + H2S с дополненными продуктами.
Решенное и коэффициентами уравнение реакции FeS + 2 HCl → FeCl2 + H2S с дополненными продуктами.
 Solved and balanced chemical equation Fe + H2S → FeS + H2 with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation Fe + H2S → FeS + H2 with completed products. Application for completing products and balancing equations.
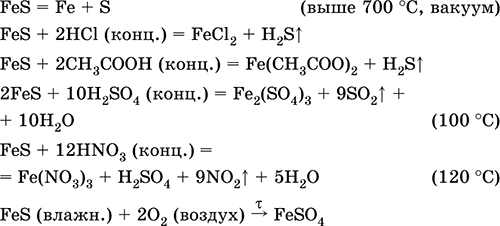 Here, we examine in more detail the nature of the temperature dependence of the reaction and combine this with experimental observations and frontier molecular orbital theory …
Here, we examine in more detail the nature of the temperature dependence of the reaction and combine this with experimental observations and frontier molecular orbital theory …
 Iron (II) sulfide or ferrous sulfide (Br.E. sulphide) is one of a family of chemical compounds and minerals with the approximate formula Fe S. Iron sulfides are often iron-deficient non …
Iron (II) sulfide or ferrous sulfide (Br.E. sulphide) is one of a family of chemical compounds and minerals with the approximate formula Fe S. Iron sulfides are often iron-deficient non …
 The exergonic reaction of FeS with H<sub>2</sub>S to form FeS<sub>2</sub> (pyrite) and H<sub>2</sub> was postulated to have operated as an early form of energy …
The exergonic reaction of FeS with H<sub>2</sub>S to form FeS<sub>2</sub> (pyrite) and H<sub>2</sub> was postulated to have operated as an early form of energy …
 Our results provide insights into a metabolic relationship that could sustain part of the deep biosphere and lend support to the iron−sulfur-world theory that postulated FeS …
Our results provide insights into a metabolic relationship that could sustain part of the deep biosphere and lend support to the iron−sulfur-world theory that postulated FeS …
 The results show a fast kinetics of FeS formation by direct reduction of H 2 S (aq) with a distinct and easily identifiable morphology, and evidence of minimum level of ferrite …
The results show a fast kinetics of FeS formation by direct reduction of H 2 S (aq) with a distinct and easily identifiable morphology, and evidence of minimum level of ferrite …
 1 FeS + 1 HCl = 1 FeCl 2 + 1 H 2 S. For each element, we check if the number of atoms is balanced on both sides of the equation. Fe is balanced: 1 atom in reagents and 1 atom in …
1 FeS + 1 HCl = 1 FeCl 2 + 1 H 2 S. For each element, we check if the number of atoms is balanced on both sides of the equation. Fe is balanced: 1 atom in reagents and 1 atom in …
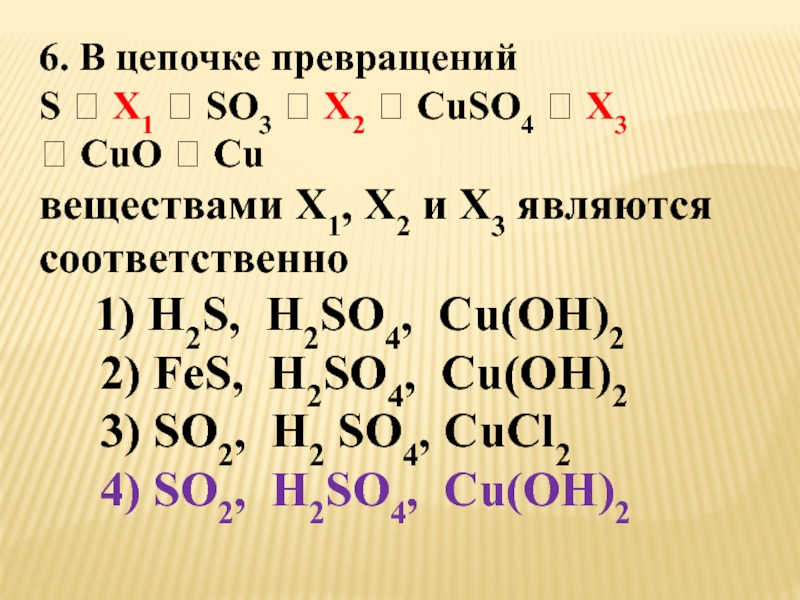
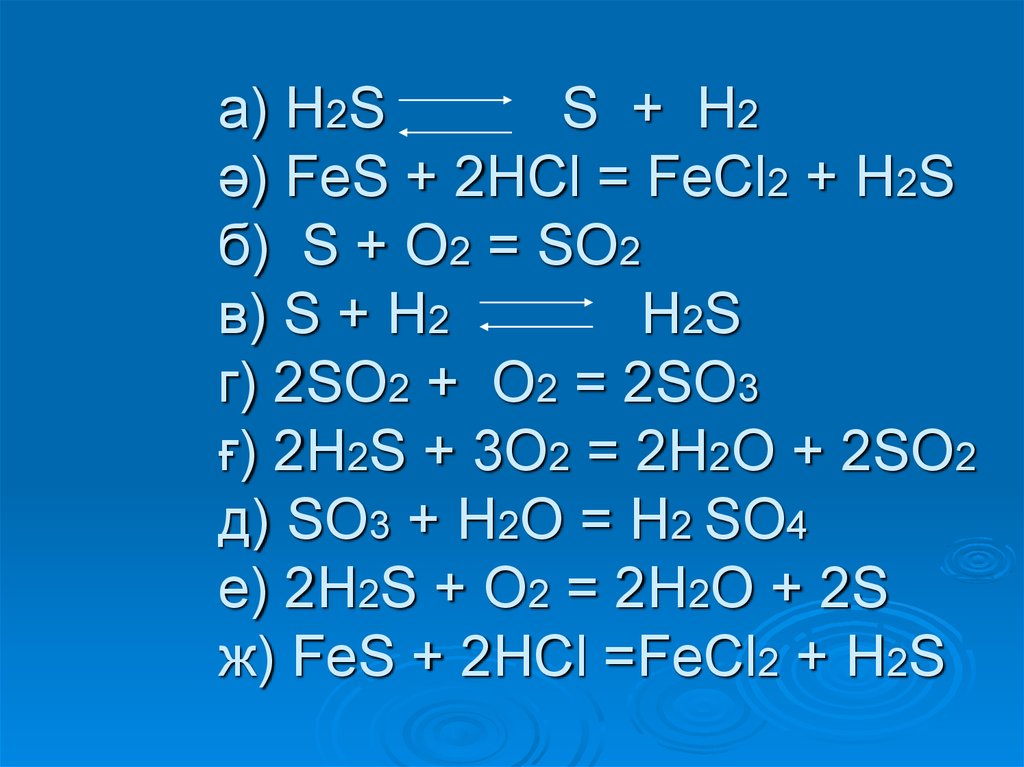
 Цепочка превращений. Уравнения химических реакций, с помощью которых можно осуществить цепочку превращений: Fe → FeCl 2 → FeS → H 2 S → NaHS → Na 2 S. – …
Цепочка превращений. Уравнения химических реакций, с помощью которых можно осуществить цепочку превращений: Fe → FeCl 2 → FeS → H 2 S → NaHS → Na 2 S. – …
 Converting H2S into H2 via a cyclic sulfur looping scheme with reactivity enhancement by incorporating low-concentration Mo-dopant into FeS.
Converting H2S into H2 via a cyclic sulfur looping scheme with reactivity enhancement by incorporating low-concentration Mo-dopant into FeS.
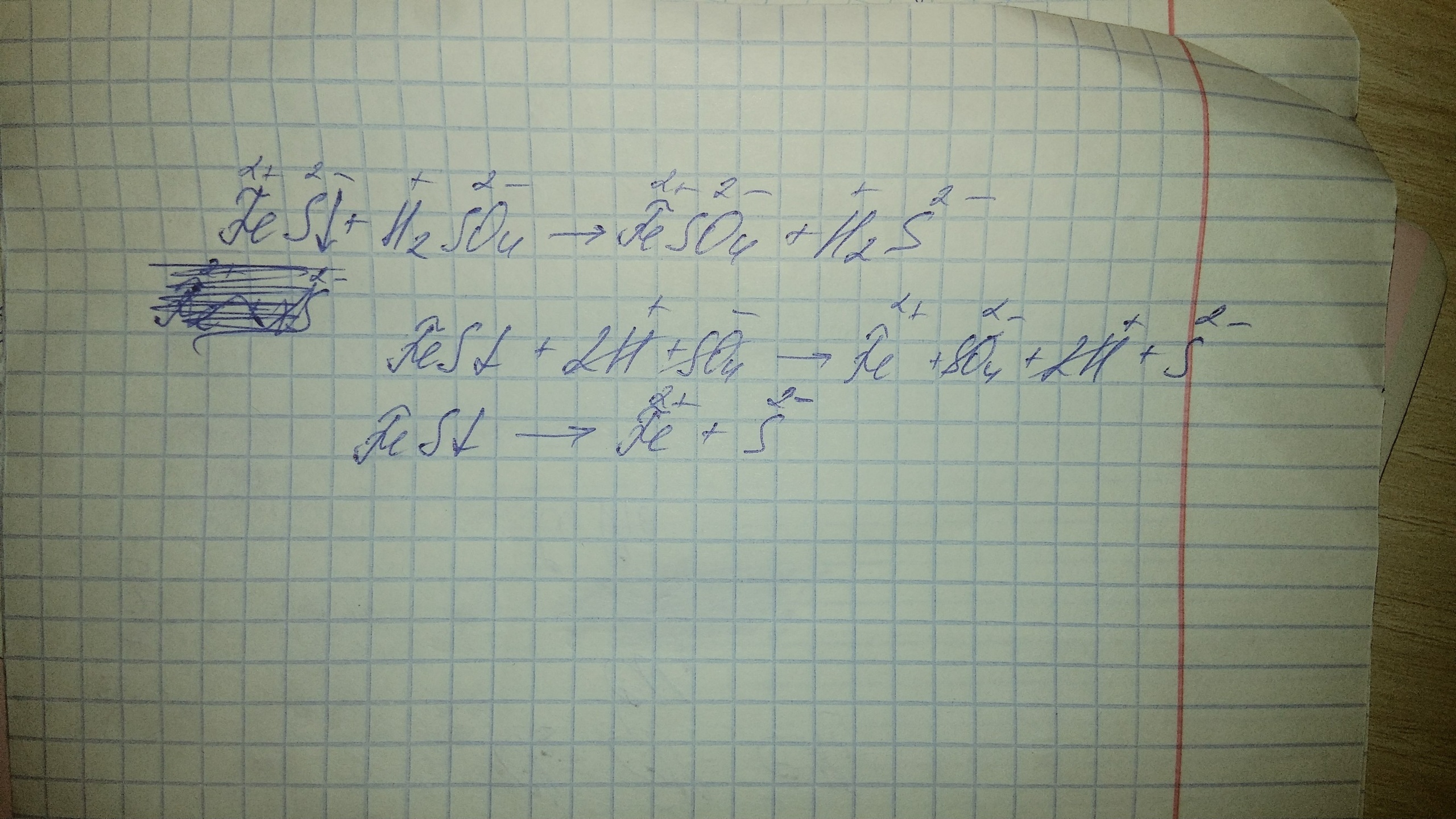 H2S – Сульфид водорода. Другие названия: Сероводород , Сернистый водород , Сероводородная вода.. показать больше. Внешность (состояние): Газ ; Бесцветный …
H2S – Сульфид водорода. Другие названия: Сероводород , Сернистый водород , Сероводородная вода.. показать больше. Внешность (состояние): Газ ; Бесцветный …
 This study enhances our understanding of structural properties of FeS 2 and the interaction of H 2 S with different FeS 2 (1 0 0) surfaces, providing guidance for developing …
This study enhances our understanding of structural properties of FeS 2 and the interaction of H 2 S with different FeS 2 (1 0 0) surfaces, providing guidance for developing …
Еще по теме:





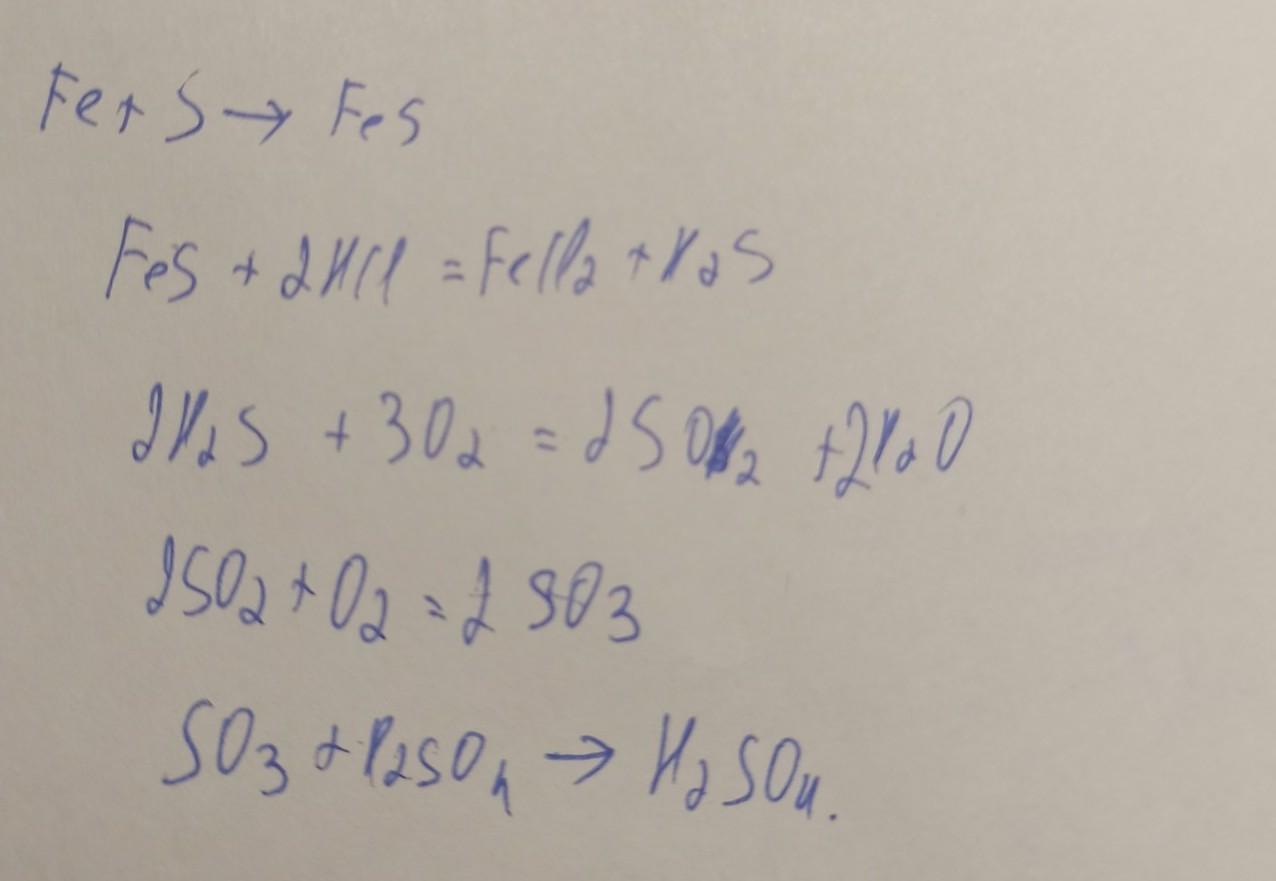



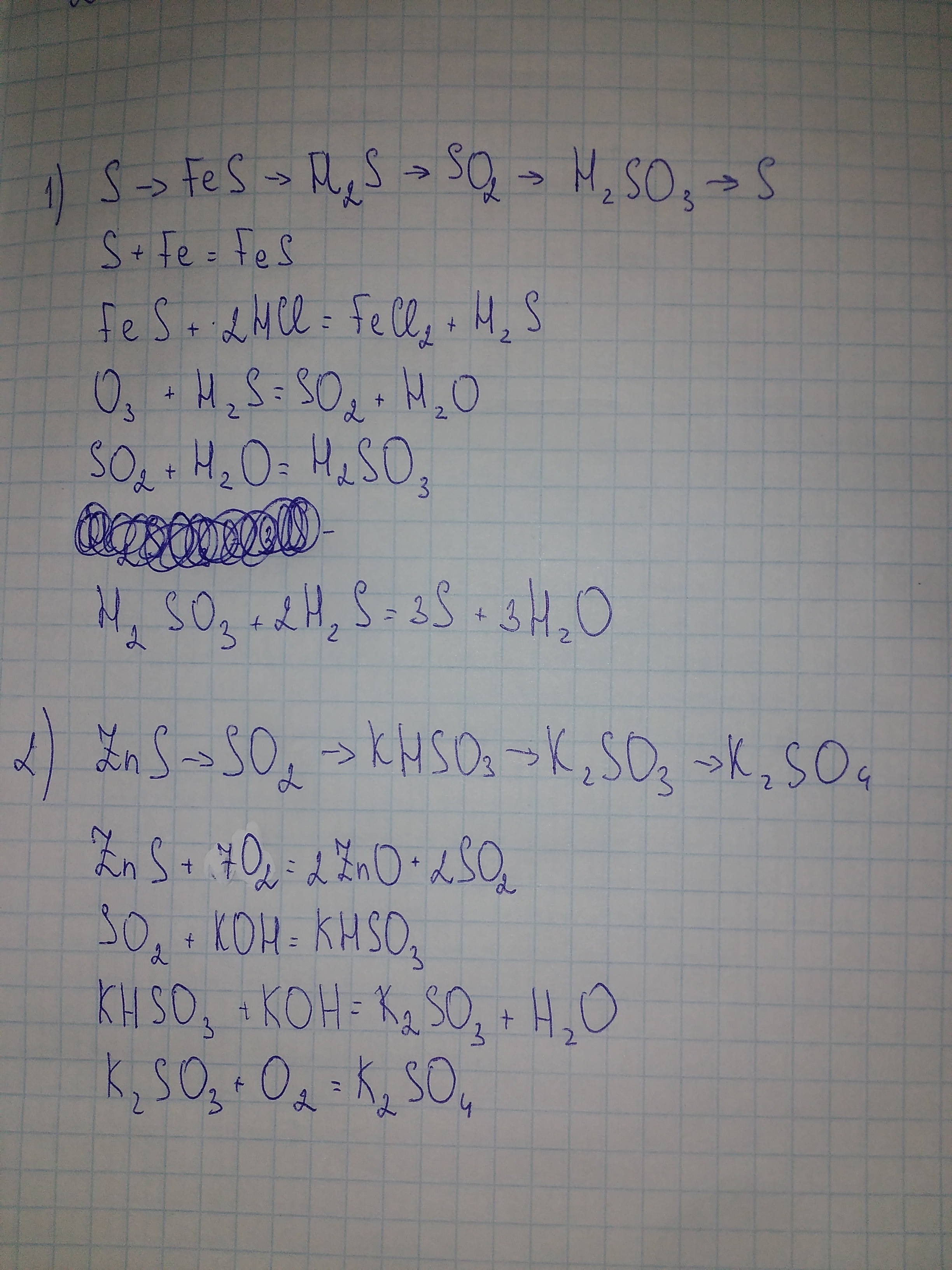


Еще по теме: