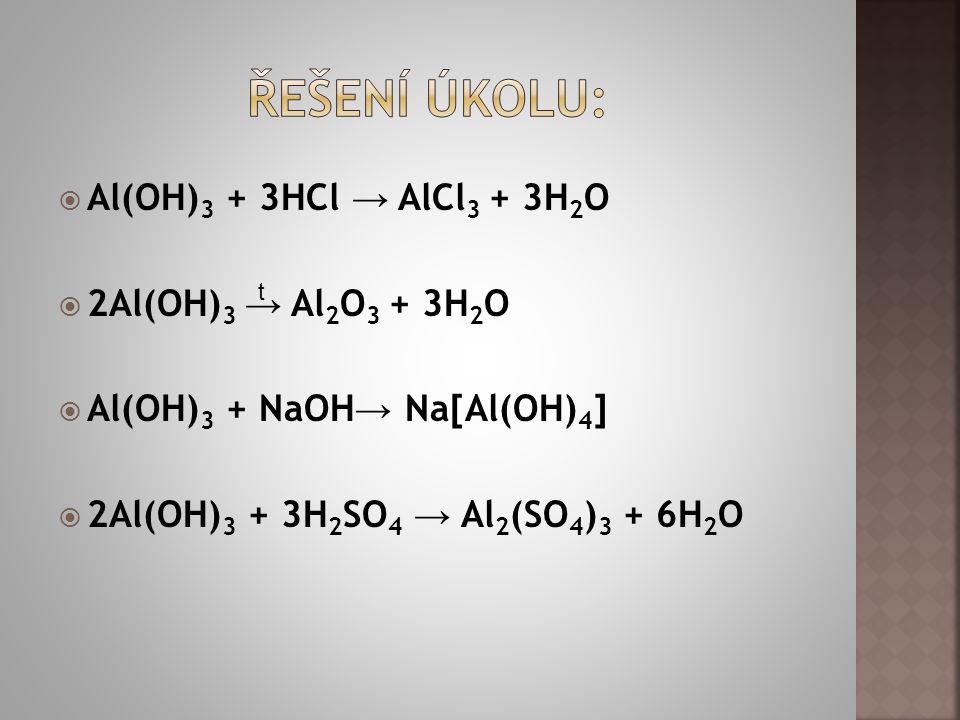
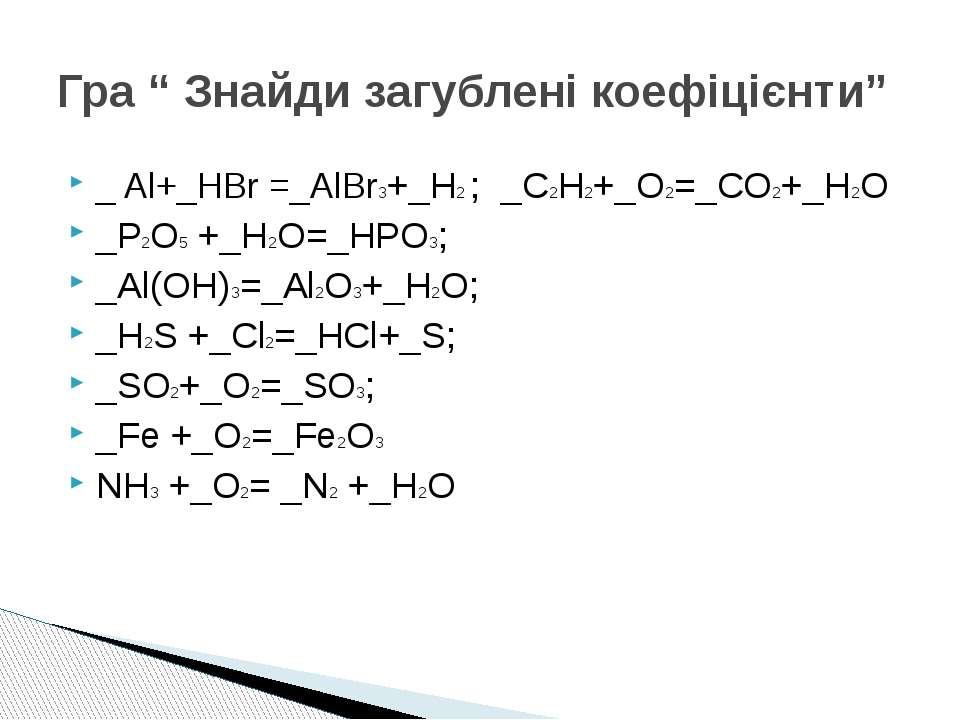
 Al (OH)3 + HBr = AlBr3 + H2O is a Double Displacement (Acid-Base) reaction where one mole of solid Aluminum Hydroxide [Al (OH) 3] and three moles of aqueous Hydrogen Bromide [HBr] …
Al (OH)3 + HBr = AlBr3 + H2O is a Double Displacement (Acid-Base) reaction where one mole of solid Aluminum Hydroxide [Al (OH) 3] and three moles of aqueous Hydrogen Bromide [HBr] …
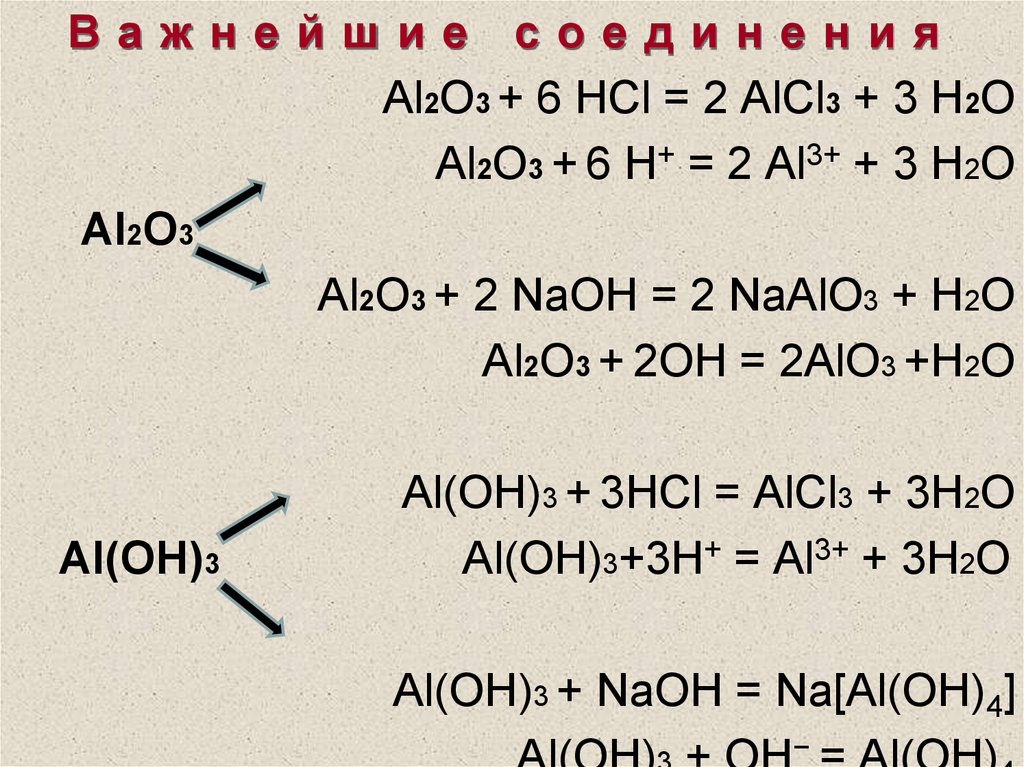
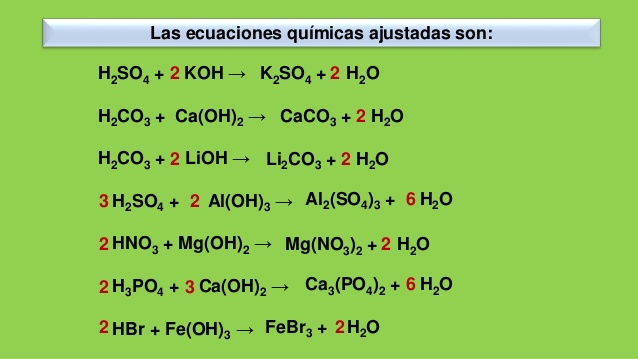
 Решенное и коэффициентами уравнение реакции Al (OH)3 + 3 HBr → AlBr3 + 3 H2O с дополненными продуктами.
Решенное и коэффициентами уравнение реакции Al (OH)3 + 3 HBr → AlBr3 + 3 H2O с дополненными продуктами.
 Multiply coefficient for HBr by 3. 1 Al (OH) 3 + 3 HBr = 1 AlBr 3 + 3 H 2 O. H is balanced: 6 atoms in reagents and 6 atoms in products. All atoms are now balanced and the whole equation is …
Multiply coefficient for HBr by 3. 1 Al (OH) 3 + 3 HBr = 1 AlBr 3 + 3 H 2 O. H is balanced: 6 atoms in reagents and 6 atoms in products. All atoms are now balanced and the whole equation is …
 3HBr+Al(OH)3→3H2O+AlBr3. Это кислотно-щелочная реакция ( нейтрализа́ция ): HBr представляет собой кислоту, Al(OH)3 является щелочным. Реактанты: HBr. Названия: …
3HBr+Al(OH)3→3H2O+AlBr3. Это кислотно-щелочная реакция ( нейтрализа́ция ): HBr представляет собой кислоту, Al(OH)3 является щелочным. Реактанты: HBr. Названия: …
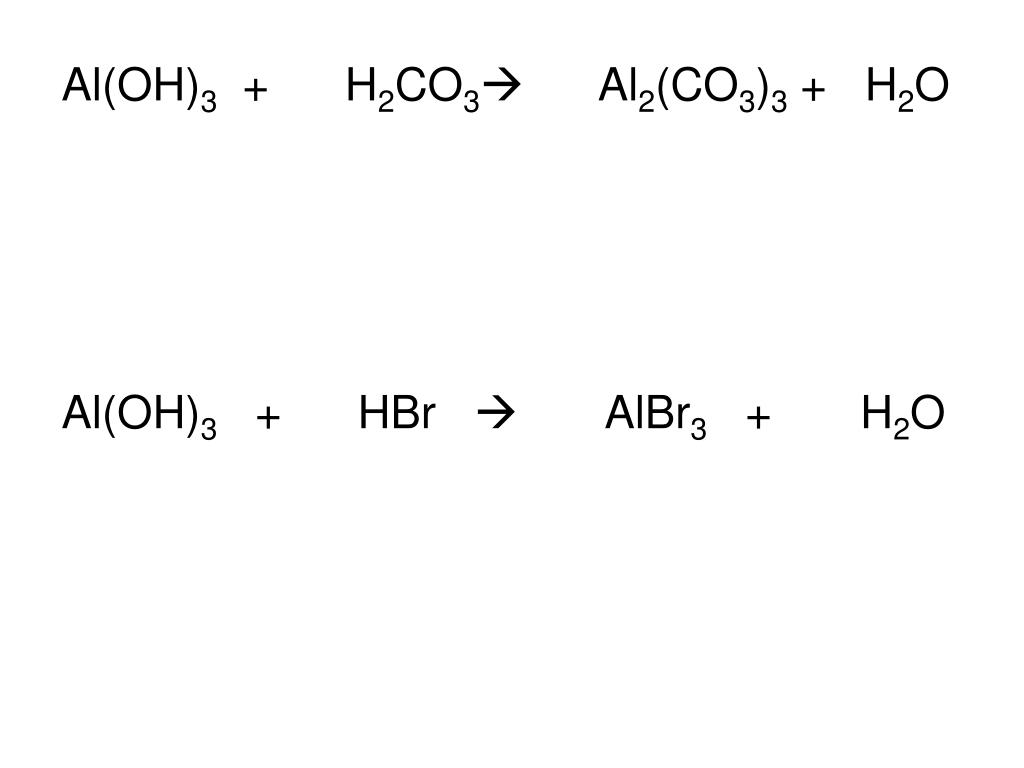 Al (OH)3 + HBr = H2O + AlBr3 is a Double Displacement (Acid-Base) reaction where one mole of solid Aluminum Hydroxide [Al (OH) 3] and three moles of aqueous Hydrogen Bromide [HBr] …
Al (OH)3 + HBr = H2O + AlBr3 is a Double Displacement (Acid-Base) reaction where one mole of solid Aluminum Hydroxide [Al (OH) 3] and three moles of aqueous Hydrogen Bromide [HBr] …
 1 Al(OH) 3 + 1 HBr = 1 AlBr 3 + 1 H(OH) For each element, we check if the number of atoms is balanced on both sides of the equation. Al is balanced: 1 atom in reagents and 1 atom in …
1 Al(OH) 3 + 1 HBr = 1 AlBr 3 + 1 H(OH) For each element, we check if the number of atoms is balanced on both sides of the equation. Al is balanced: 1 atom in reagents and 1 atom in …
 Расширенный поиск. Или Попробуйте случайную реакцию. Приложение для вычисления и дополнения продуктов реакции. Ионные и окислительно-восстановительные реакции!
Расширенный поиск. Или Попробуйте случайную реакцию. Приложение для вычисления и дополнения продуктов реакции. Ионные и окислительно-восстановительные реакции!
 HBr + Al (OH)3 = H2O + AlBr3 is a Double Displacement (Acid-Base) reaction where three moles of aqueous Hydrogen Bromide [HBr] and one mole of solid Aluminum Hydroxide [Al (OH) 3] …
HBr + Al (OH)3 = H2O + AlBr3 is a Double Displacement (Acid-Base) reaction where three moles of aqueous Hydrogen Bromide [HBr] and one mole of solid Aluminum Hydroxide [Al (OH) 3] …
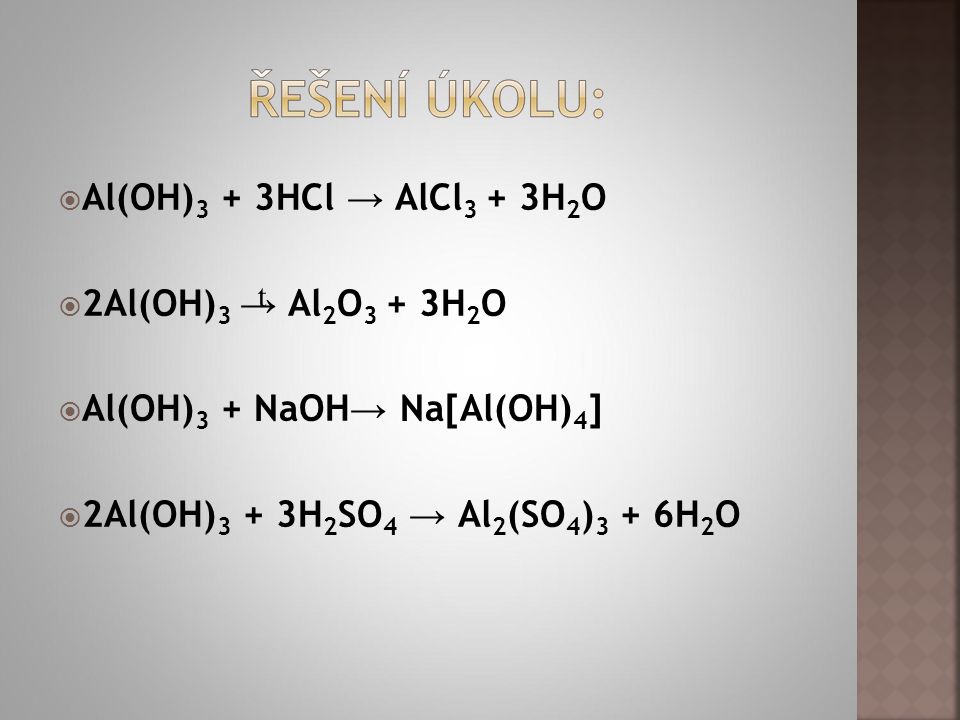 Multiply coefficient for H 2 O by 3. 3 HBr + 1 Al (OH) 3 = 3 H 2 O + 1 AlBr 3. H is balanced: 6 atoms in reagents and 6 atoms in products. All atoms are now balanced and the whole …
Multiply coefficient for H 2 O by 3. 3 HBr + 1 Al (OH) 3 = 3 H 2 O + 1 AlBr 3. H is balanced: 6 atoms in reagents and 6 atoms in products. All atoms are now balanced and the whole …
Еще по теме:





Еще по теме: