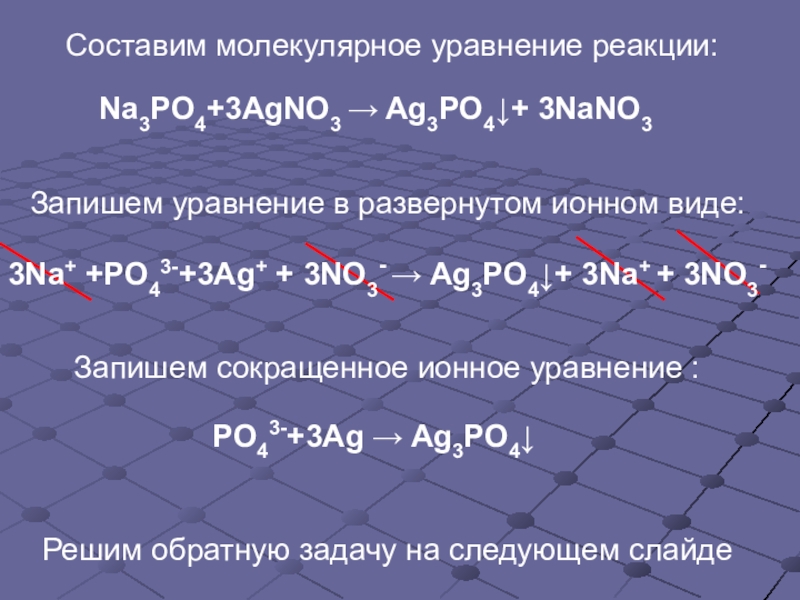
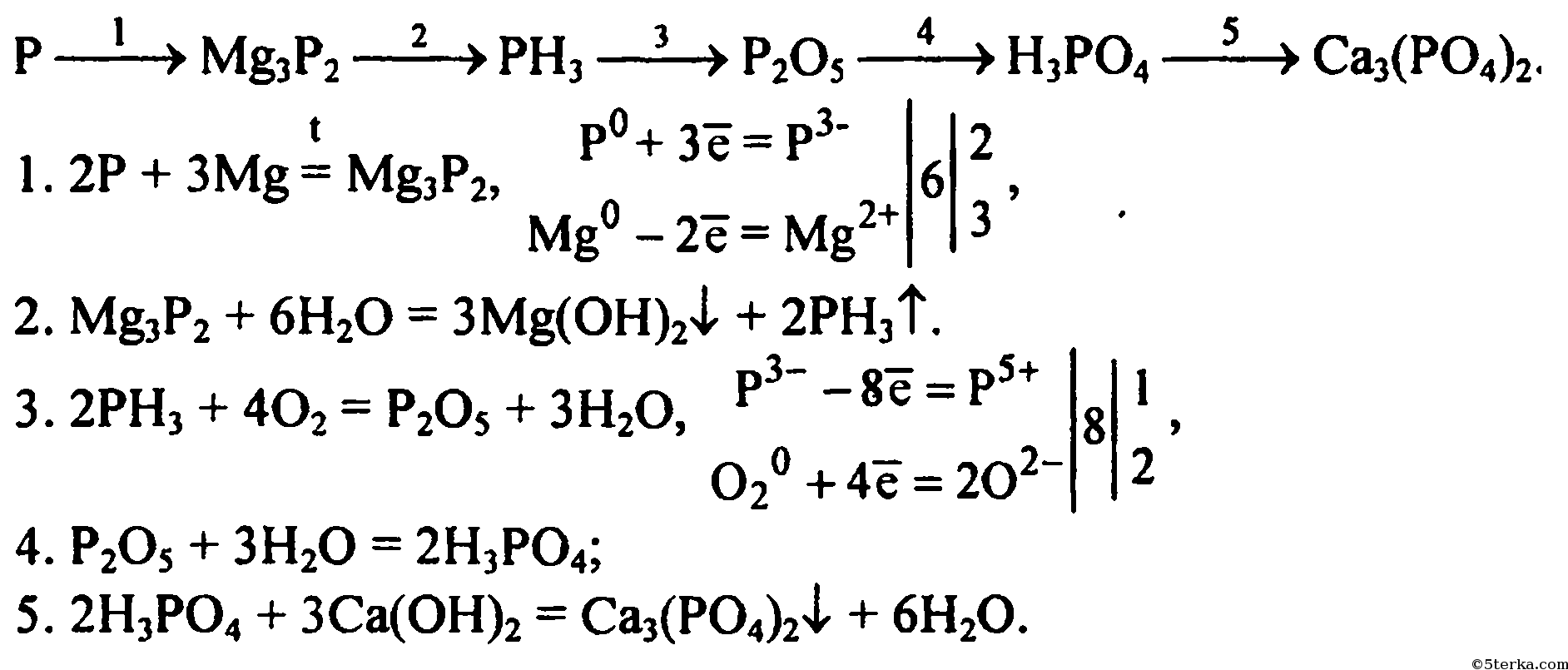Решенное и коэффициентами уравнение реакции 3 HCl + Na3PO4 → 3 NaCl + H3PO4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 3 HCl + Na3PO4 → 3 NaCl + H3PO4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Na3PO4+HCl=Na2HPO4+NaCl( кислота концентрированная должна быть вроде)
Na3PO4+HCl=Na2HPO4+NaCl( кислота концентрированная должна быть вроде)
 Solved and balanced chemical equation 3 NaCl + H3PO4 → 3 HCl + Na3PO4 with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation 3 NaCl + H3PO4 → 3 HCl + Na3PO4 with completed products. Application for completing products and balancing equations.
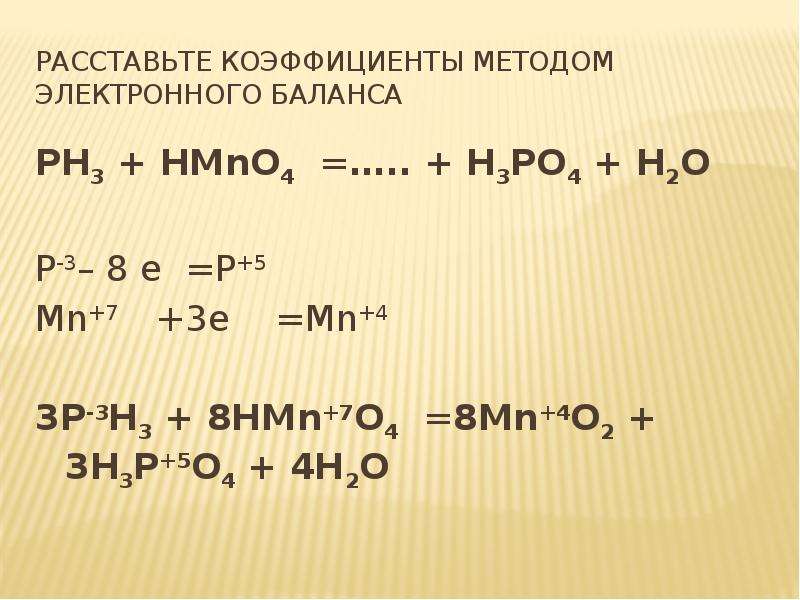
 Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 Na 3 PO 4 + 1 HCl = 1 NaCl + 1 H 3 PO 4. For each element, we check if the number of atoms is …
Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 Na 3 PO 4 + 1 HCl = 1 NaCl + 1 H 3 PO 4. For each element, we check if the number of atoms is …
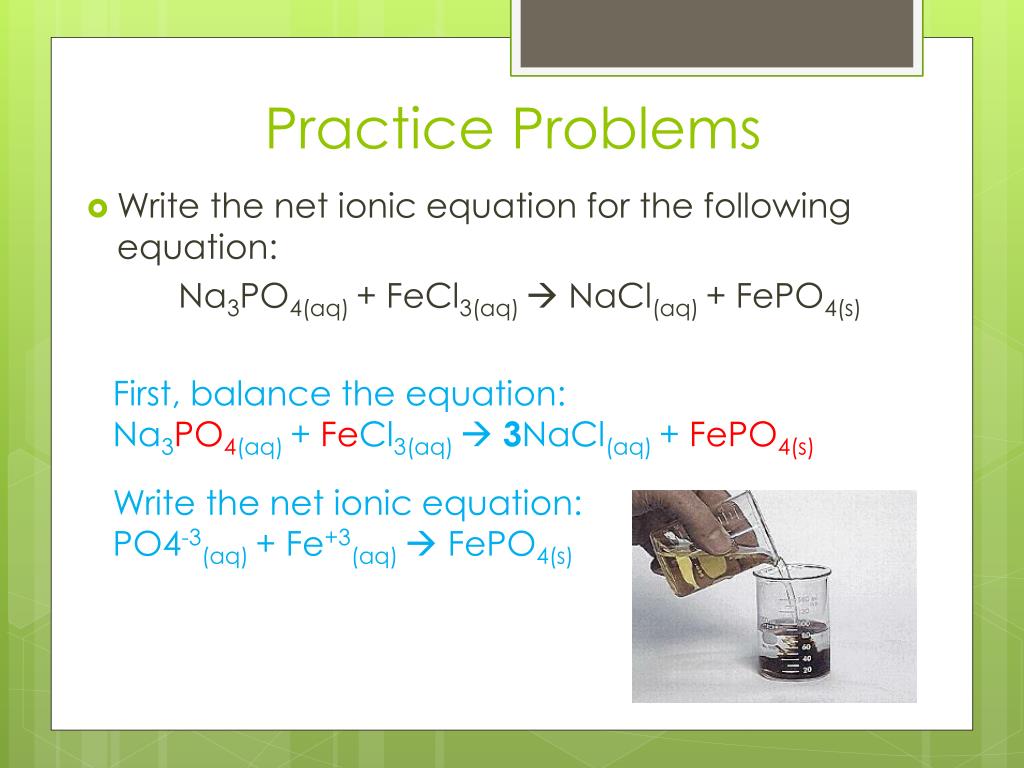
 Calculate the complete and net ionic equations, spectators and precipitates in H3PO4(aq) + 3NaCl(aq) = 3HCl(aq) + Na3PO4(aq)
Calculate the complete and net ionic equations, spectators and precipitates in H3PO4(aq) + 3NaCl(aq) = 3HCl(aq) + Na3PO4(aq)
![Khi trộn Na3PO4 vào dung dịch HCl [đã giải] – Học Hóa Online](https://image3.slideserve.com/5916703/slide18-l.jpg) Câu trả lời tốt nhất. Nguyễn Xuân Phúc ở bài Cho các phát biểu sau: (1) Methylamine, dimethylamine, trimethylamine và ethylamine là những chất khí mùi khai khó …
Câu trả lời tốt nhất. Nguyễn Xuân Phúc ở bài Cho các phát biểu sau: (1) Methylamine, dimethylamine, trimethylamine và ethylamine là những chất khí mùi khai khó …
 Решенное и коэффициентами уравнение реакции 3 NaCl + H3PO4 → 3 HCl + Na3PO4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 3 NaCl + H3PO4 → 3 HCl + Na3PO4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Therefore, the balanced chemical equation is Na3PO4 + 3HCl → H3PO4 + 3NaCl. Na3PO4+3HCl —> H3PO4+3NaCl. The reaction type for this equation is a double …
Therefore, the balanced chemical equation is Na3PO4 + 3HCl → H3PO4 + 3NaCl. Na3PO4+3HCl —> H3PO4+3NaCl. The reaction type for this equation is a double …
 Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
 A 1.200 M solution of HCl is added to a solution containing 0.150 moles of sodium phosphate (Na3PO4). The resulting solution is then diluted to exactly 0.500 L, at which time the pH was …
A 1.200 M solution of HCl is added to a solution containing 0.150 moles of sodium phosphate (Na3PO4). The resulting solution is then diluted to exactly 0.500 L, at which time the pH was …
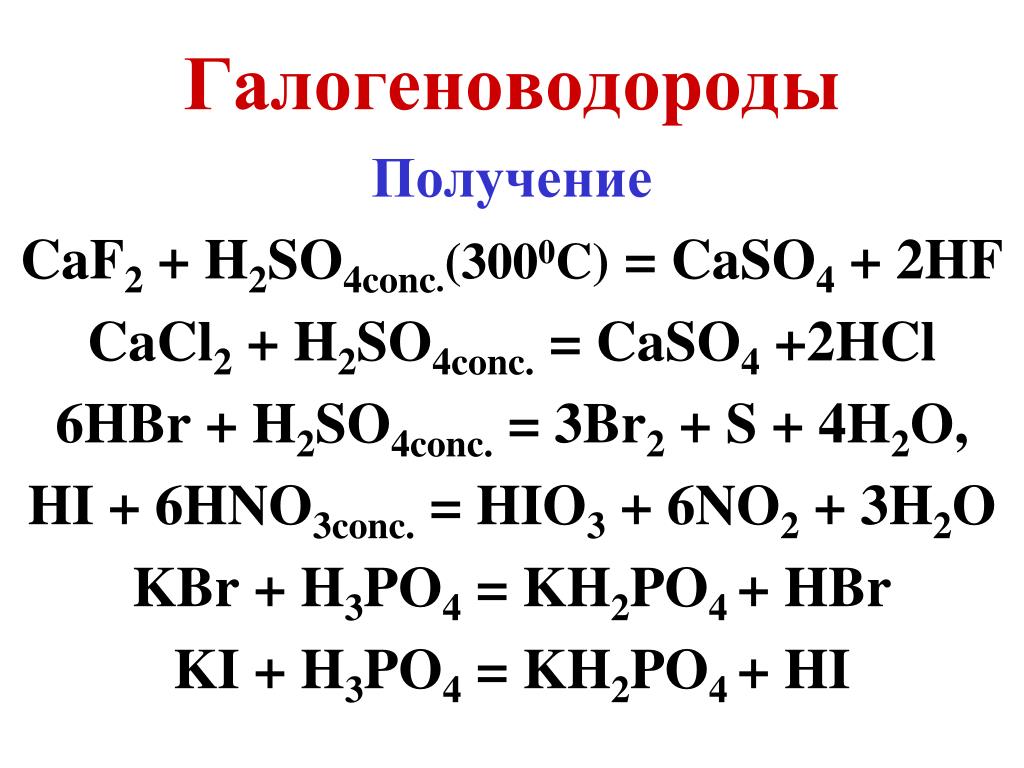
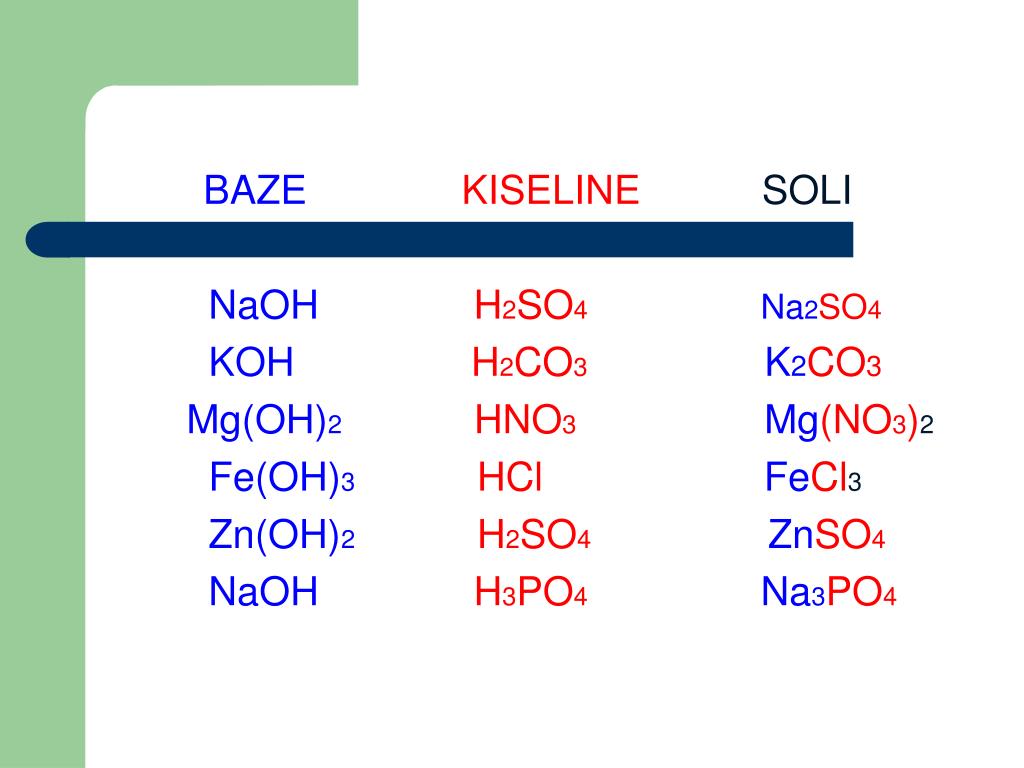
 Na3PO4 + HCl = NaH2PO4 + NaCl is a Double Displacement (Metathesis) reaction where one mole of aqueous Trisodium Phosphate [Na 3 PO 4] and two moles of aqueous Hydrogen …
Na3PO4 + HCl = NaH2PO4 + NaCl is a Double Displacement (Metathesis) reaction where one mole of aqueous Trisodium Phosphate [Na 3 PO 4] and two moles of aqueous Hydrogen …
 Ионное уравнение реакции взаимодействия ортофосфорной кислоты (H 3 PO 4) и хлорида натрия (NaCl) имеет следующий вид: H 3 PO 4 + 3 NaCl = 3 HCl + Na 3 PO 4; …
Ионное уравнение реакции взаимодействия ортофосфорной кислоты (H 3 PO 4) и хлорида натрия (NaCl) имеет следующий вид: H 3 PO 4 + 3 NaCl = 3 HCl + Na 3 PO 4; …
 Chemistry questions and answers. Sodium phosphate, Na3PO4, reacts with HCl to form H3PO4 and NaCl. How many liters of 0.1 M HCl (aq) are required to react with 0.1 mole of sodium …
Chemistry questions and answers. Sodium phosphate, Na3PO4, reacts with HCl to form H3PO4 and NaCl. How many liters of 0.1 M HCl (aq) are required to react with 0.1 mole of sodium …
 1 NaCl + 1 H 3 PO 4 = 1 HCl + 1 Na 3 PO 4 For each element, we check if the number of atoms is balanced on both sides of the equation. Na is not balanced: 1 atom in reagents and 3 atoms in …
1 NaCl + 1 H 3 PO 4 = 1 HCl + 1 Na 3 PO 4 For each element, we check if the number of atoms is balanced on both sides of the equation. Na is not balanced: 1 atom in reagents and 3 atoms in …
Еще по теме:




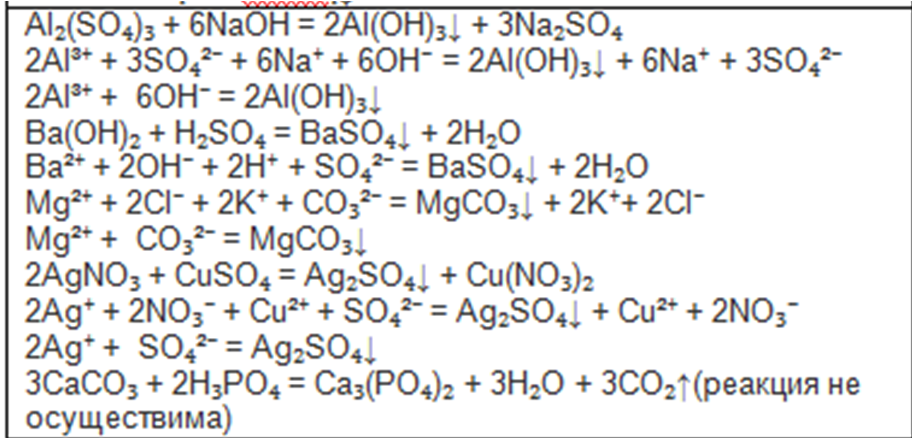




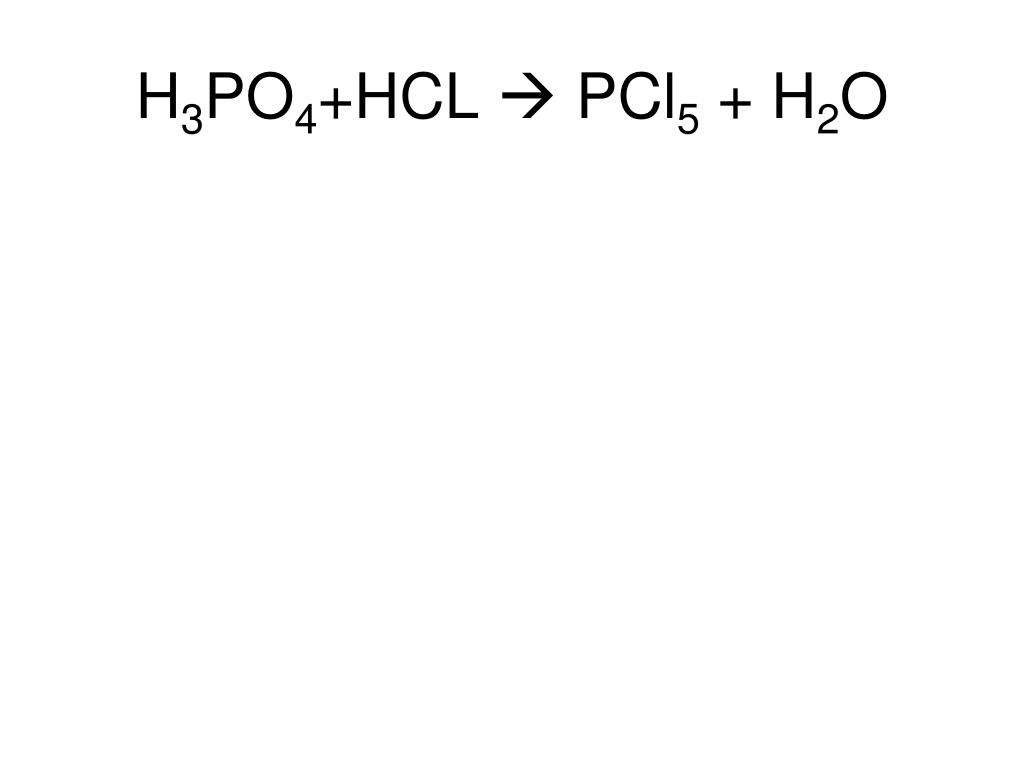


Еще по теме: