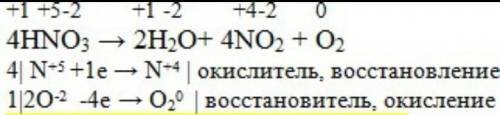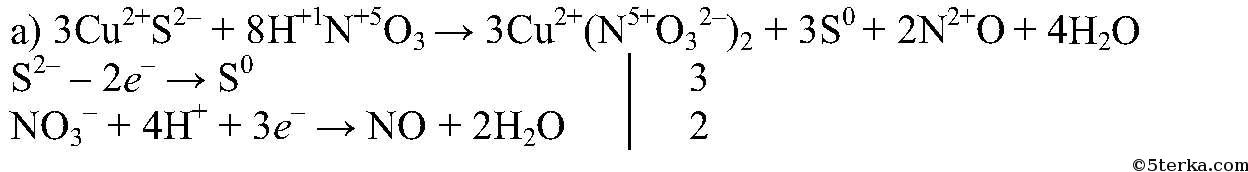Balance the reaction of H2S + HNO3 = S + NO + H2O using this chemical equation balancer!
Balance the reaction of H2S + HNO3 = S + NO + H2O using this chemical equation balancer!
 H2S является восстановителем, HNO3 является окислителем. Решенное и коэффициентами уравнение реакции 8 HNO3 + H2S → H2SO4 + 8 NO2 + 4 H2O с …
H2S является восстановителем, HNO3 является окислителем. Решенное и коэффициентами уравнение реакции 8 HNO3 + H2S → H2SO4 + 8 NO2 + 4 H2O с …
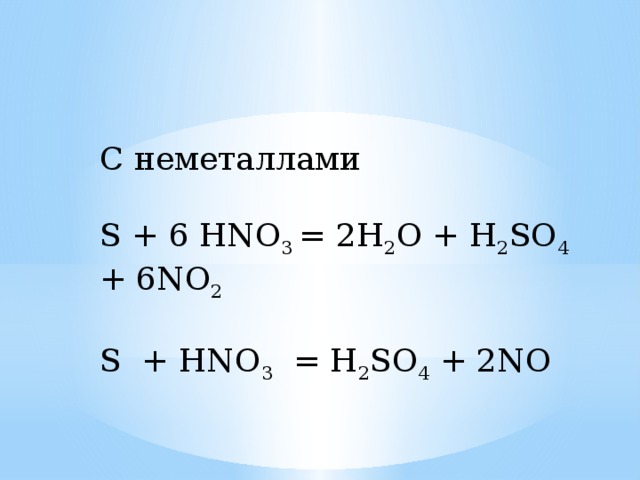 Химическое уравнение представляет собой химическую реакцию. На нем показаны реагенты (вещества, которые начинают реакцию) и продукты (вещества, образующиеся …
Химическое уравнение представляет собой химическую реакцию. На нем показаны реагенты (вещества, которые начинают реакцию) и продукты (вещества, образующиеся …
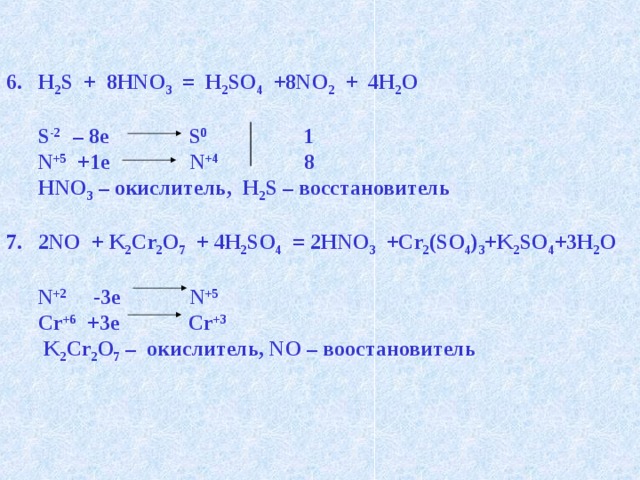
 H2S является восстановителем, HNO3 является окислителем. Решенное и коэффициентами уравнение реакции 8 HNO3 + 3 H2S → 3 H2SO4 + 8 NO + 4 H2O с …
H2S является восстановителем, HNO3 является окислителем. Решенное и коэффициентами уравнение реакции 8 HNO3 + 3 H2S → 3 H2SO4 + 8 NO + 4 H2O с …
 Решенное и коэффициентами уравнение реакции 2 HNO3 + H2S → S + 2 NO2 + 2 H2O с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 HNO3 + H2S → S + 2 NO2 + 2 H2O с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
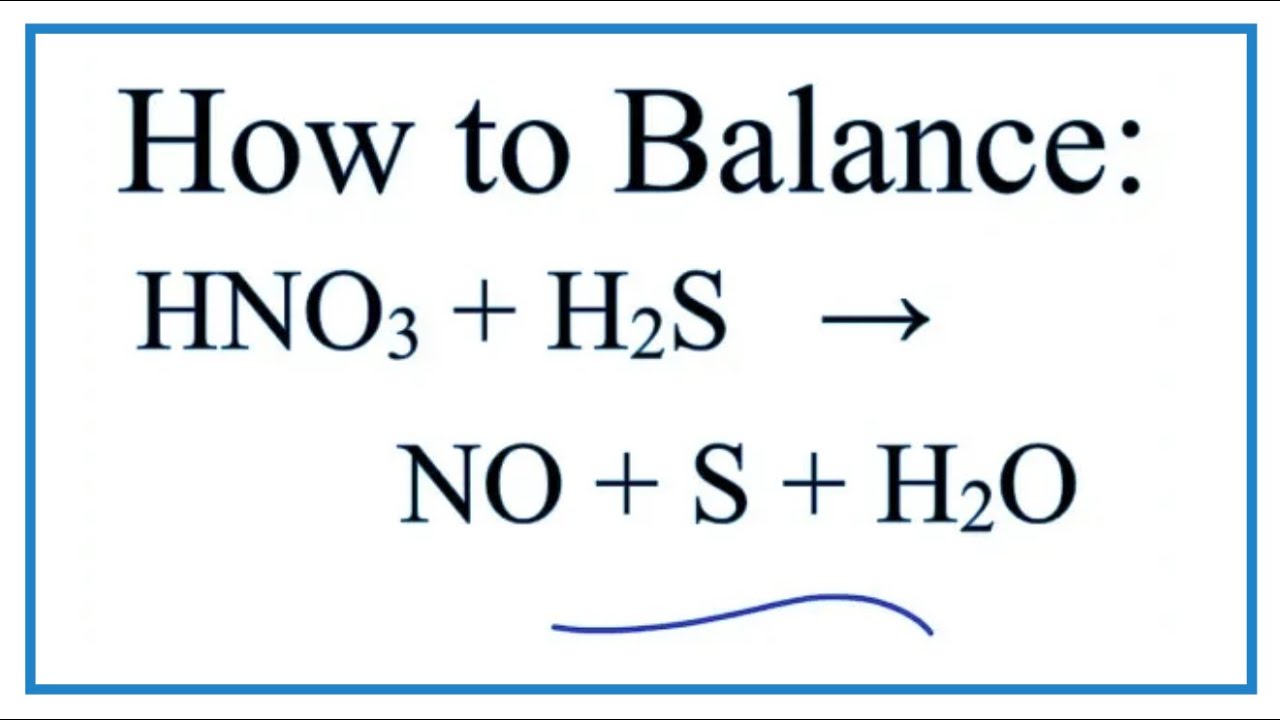
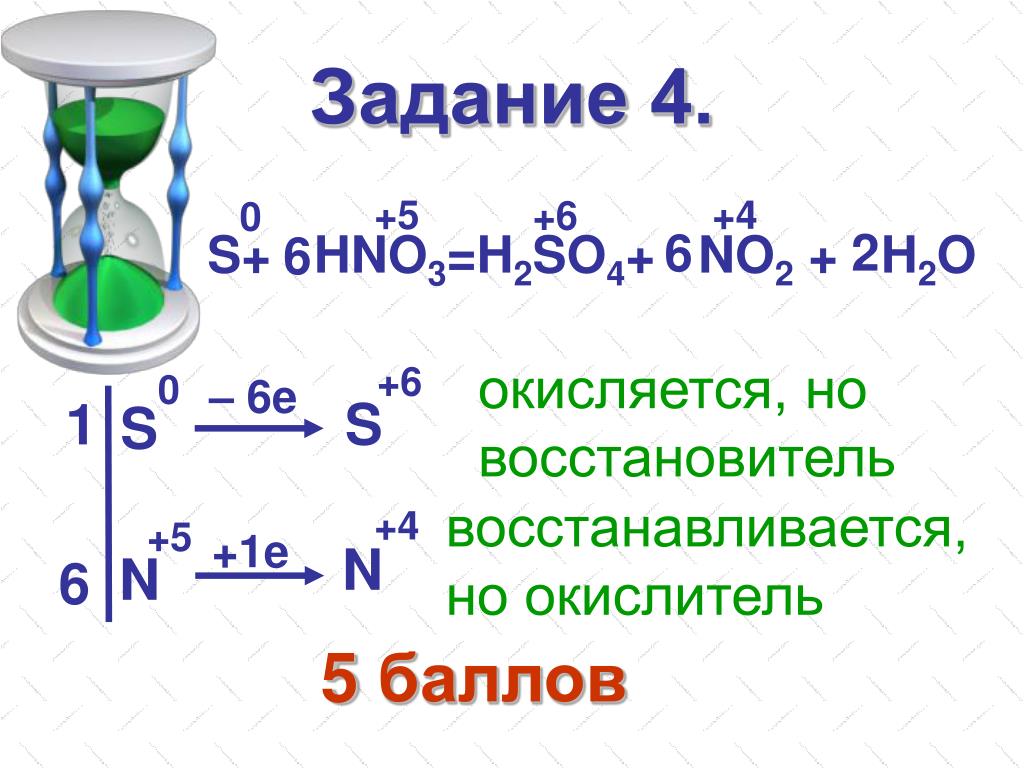 In this video we'll balance the equation HNO3 + H2S = NO + S + H2O and provide the correct coefficients for each compound..more. Note: to balance as a redox reaction, the steps are provided.
In this video we'll balance the equation HNO3 + H2S = NO + S + H2O and provide the correct coefficients for each compound..more. Note: to balance as a redox reaction, the steps are provided.
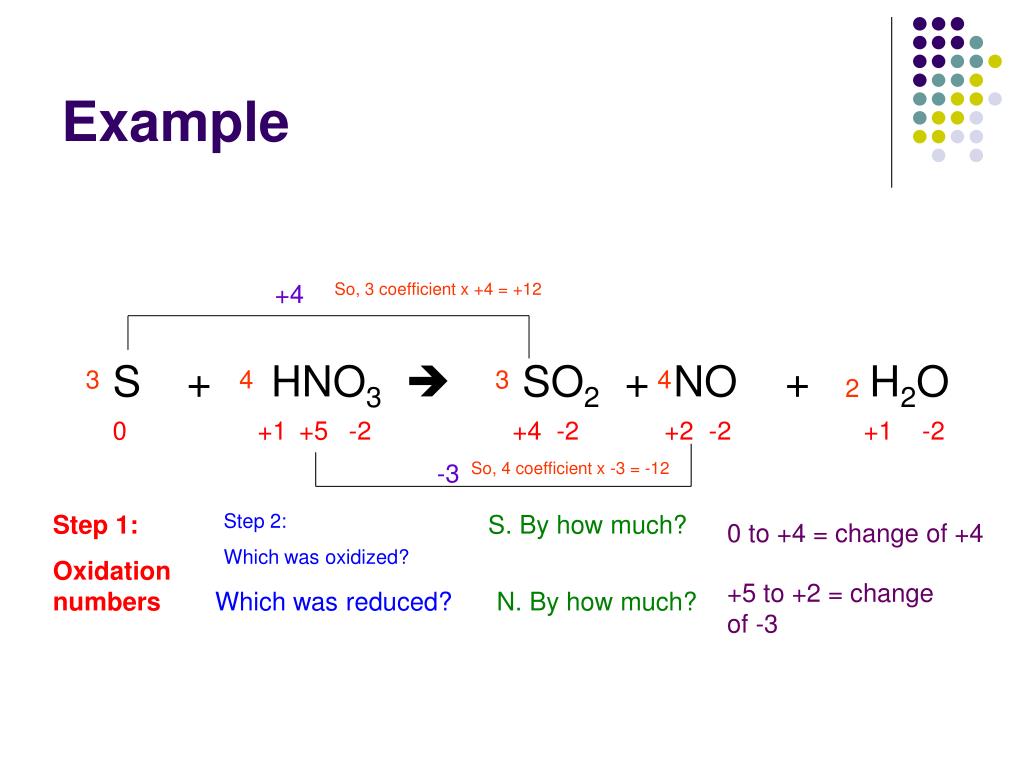 Direct link to this balanced equation: Please tell about this free chemistry software to your friends! Instructions on balancing chemical equations:
Direct link to this balanced equation: Please tell about this free chemistry software to your friends! Instructions on balancing chemical equations:
 To be balanced, every element in H2S + HNO3 = H2SO4 + NO + H2O must have the same number of atoms on each side of the equation. When using the inspection method (also known …
To be balanced, every element in H2S + HNO3 = H2SO4 + NO + H2O must have the same number of atoms on each side of the equation. When using the inspection method (also known …
 Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. The answer will appear below; Always use the upper case for the first character in …
Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. The answer will appear below; Always use the upper case for the first character in …
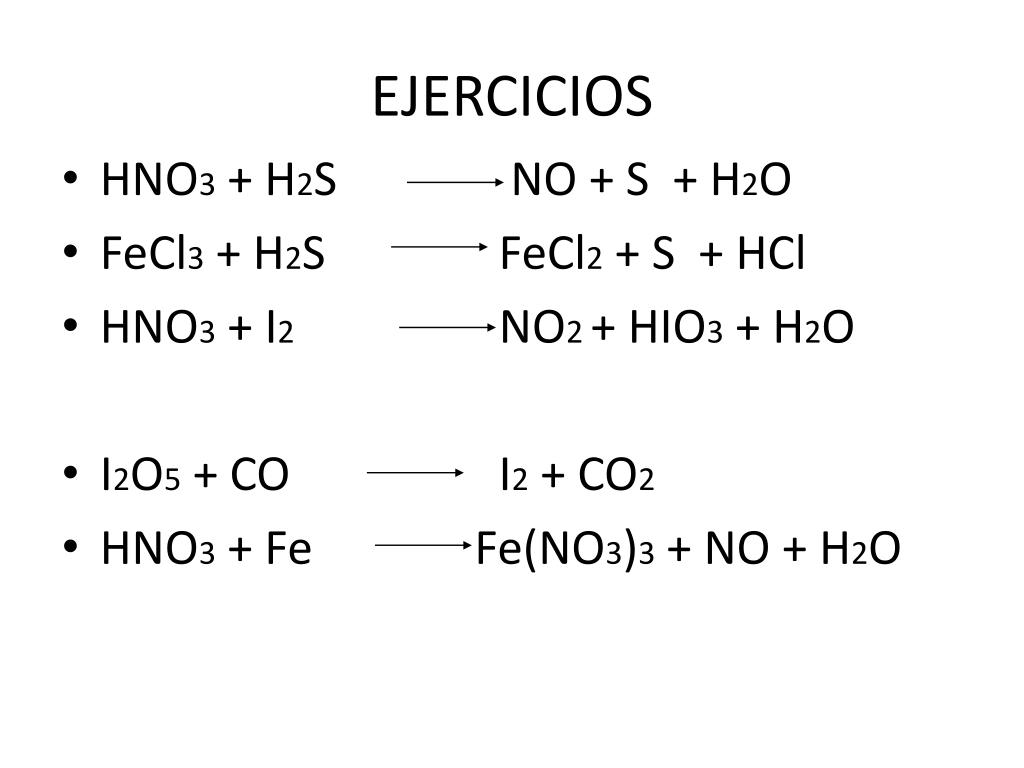 Balance the reaction of H2S + HNO3 = NO + S + H2O using this chemical equation balancer!
Balance the reaction of H2S + HNO3 = NO + S + H2O using this chemical equation balancer!
 Balance the reaction of HNO3 + H2S = NO + S + H2O using this chemical equation balancer!
Balance the reaction of HNO3 + H2S = NO + S + H2O using this chemical equation balancer!
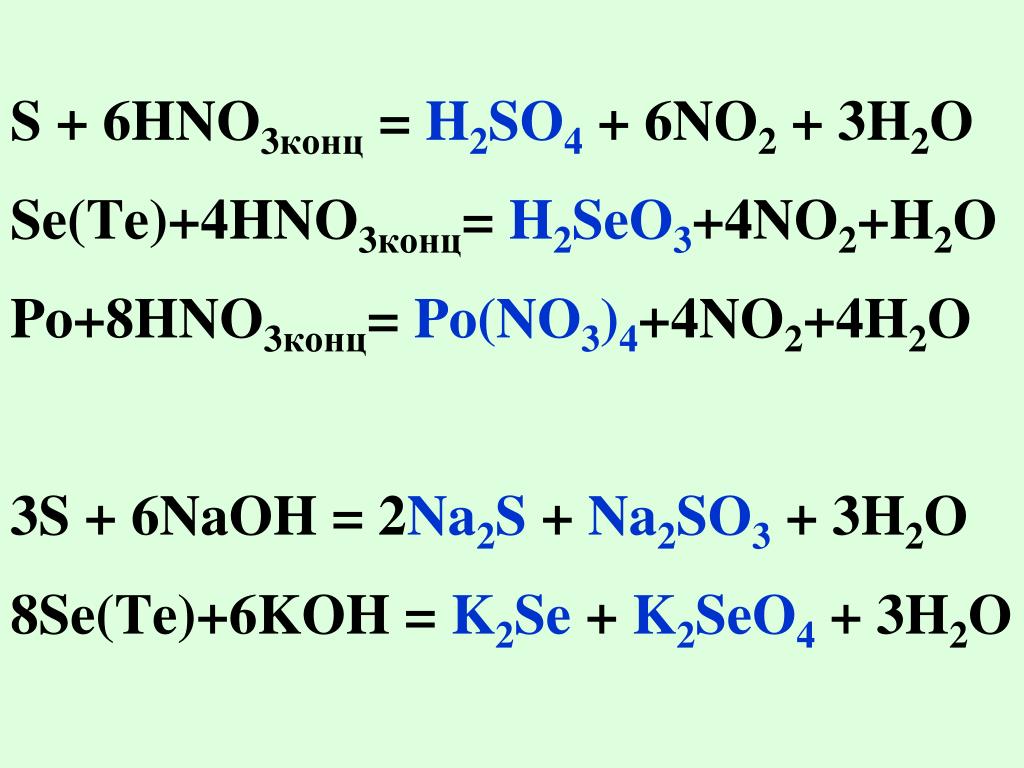 According to Ref.1, the reaction of $\ce{HNO3}$ with $\ce{H2S}$ has been processed in multi steps, which agrees with Maurice's suggestion in the first answer. The …
According to Ref.1, the reaction of $\ce{HNO3}$ with $\ce{H2S}$ has been processed in multi steps, which agrees with Maurice's suggestion in the first answer. The …
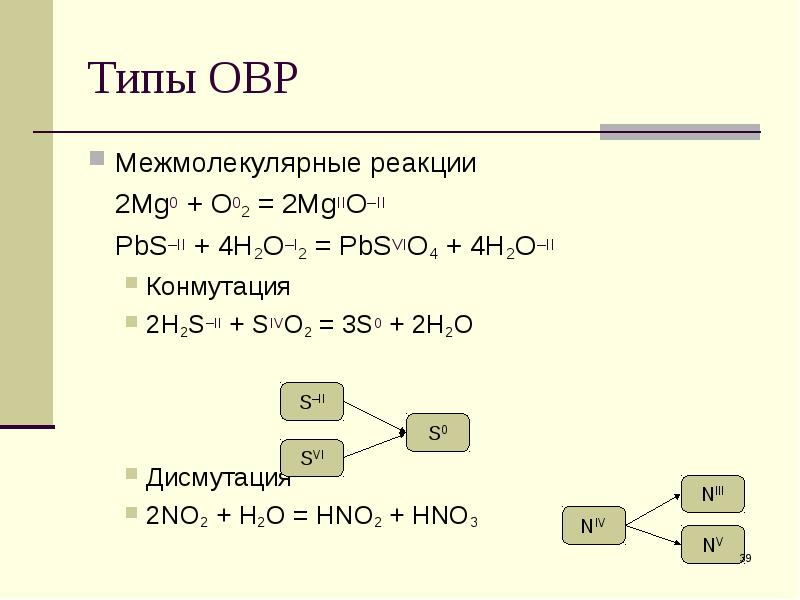
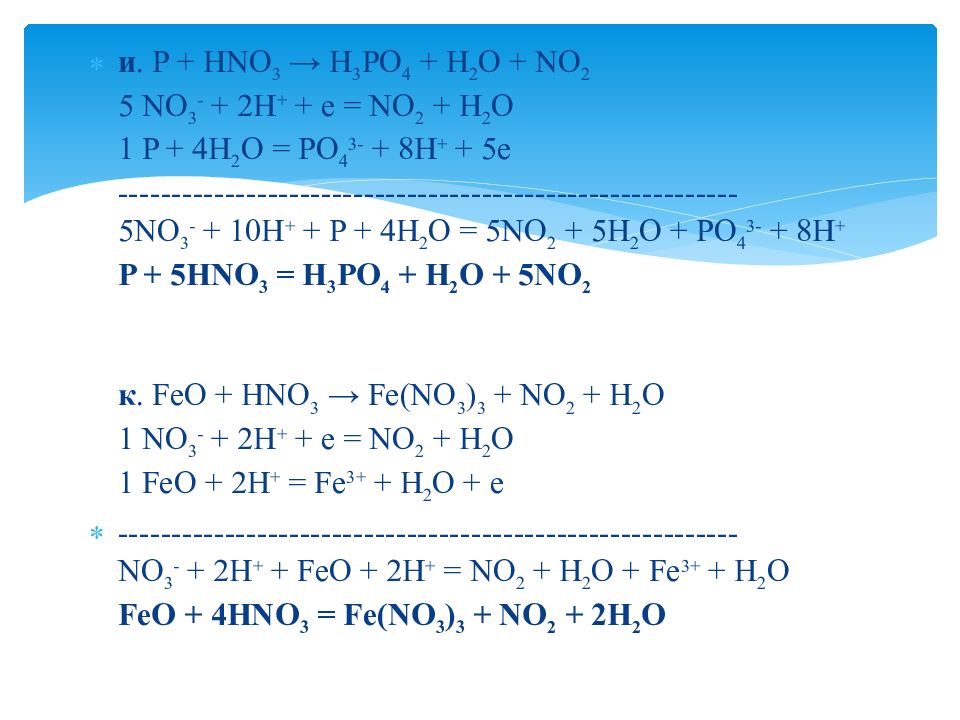
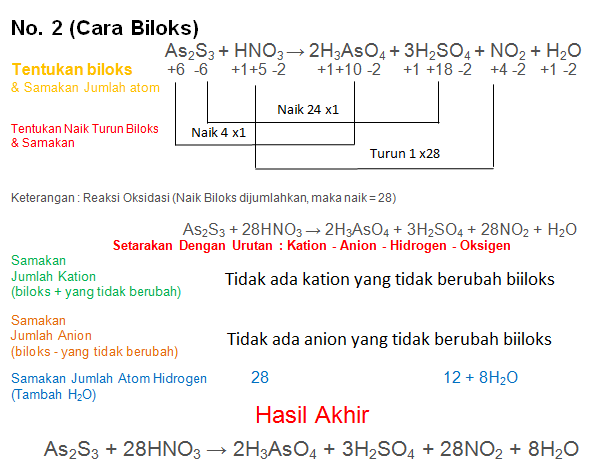
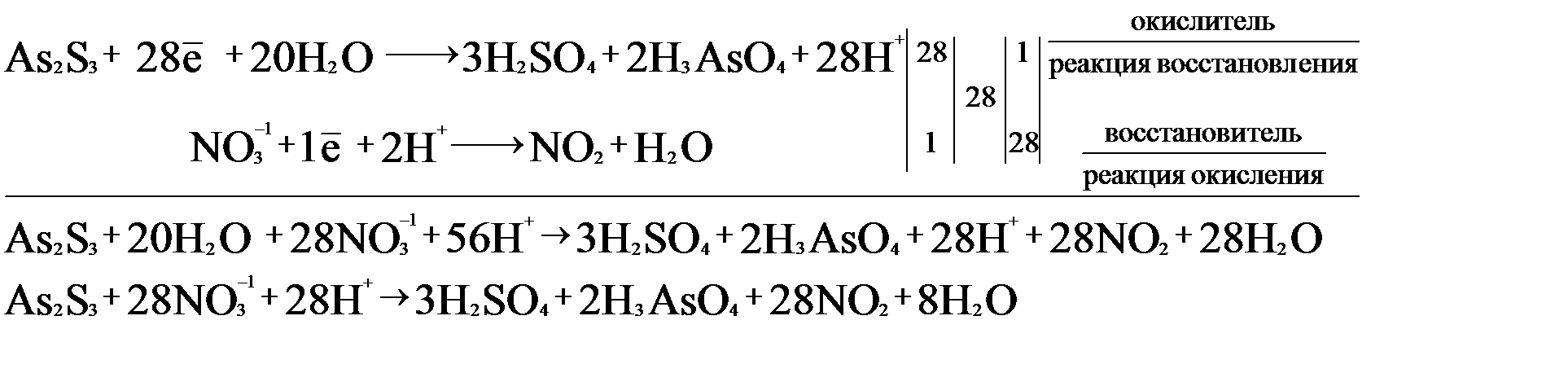
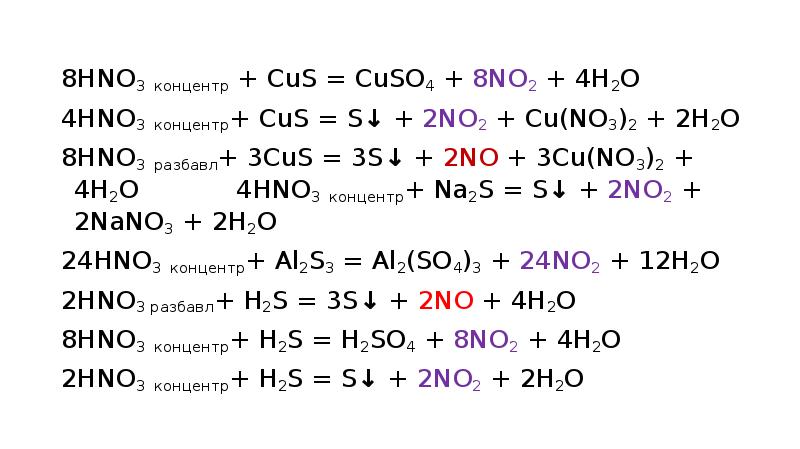 In the oxidation number change method the underlying principle is that the gain in the oxidation number (number of electrons) in one reactant must be equal to the loss in the oxidation …
In the oxidation number change method the underlying principle is that the gain in the oxidation number (number of electrons) in one reactant must be equal to the loss in the oxidation …
 There are 2 H atoms on the left and 2 H atom on the right. There are 2 O atoms on the left and 1 O atom on the right. Check the balance. Now, both sides have 4 H atoms and 2 O atoms. The …
There are 2 H atoms on the left and 2 H atom on the right. There are 2 O atoms on the left and 1 O atom on the right. Check the balance. Now, both sides have 4 H atoms and 2 O atoms. The …
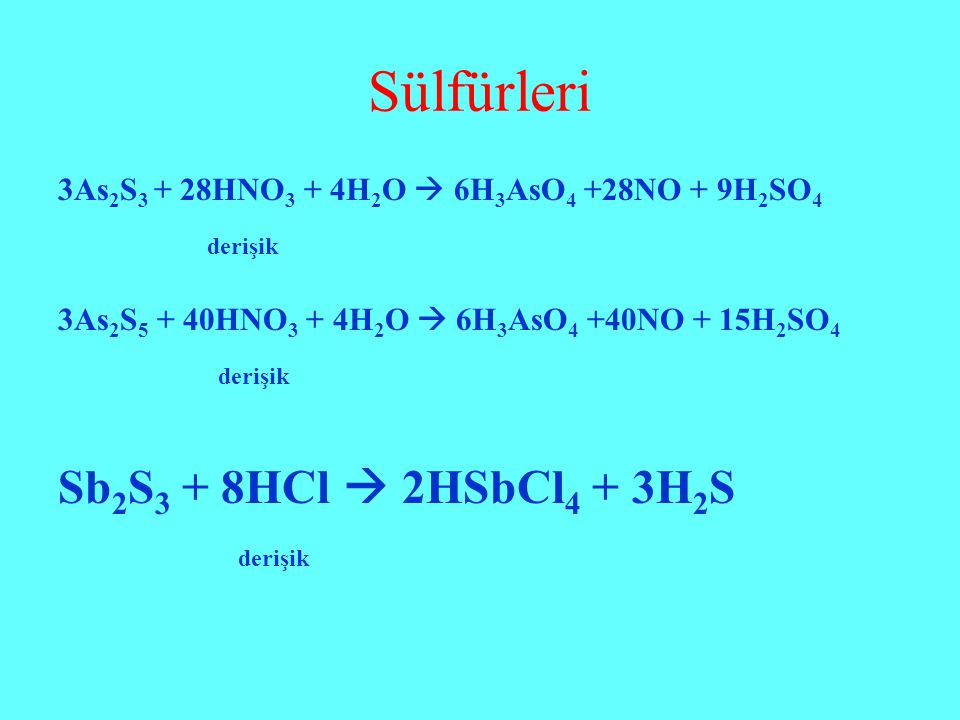
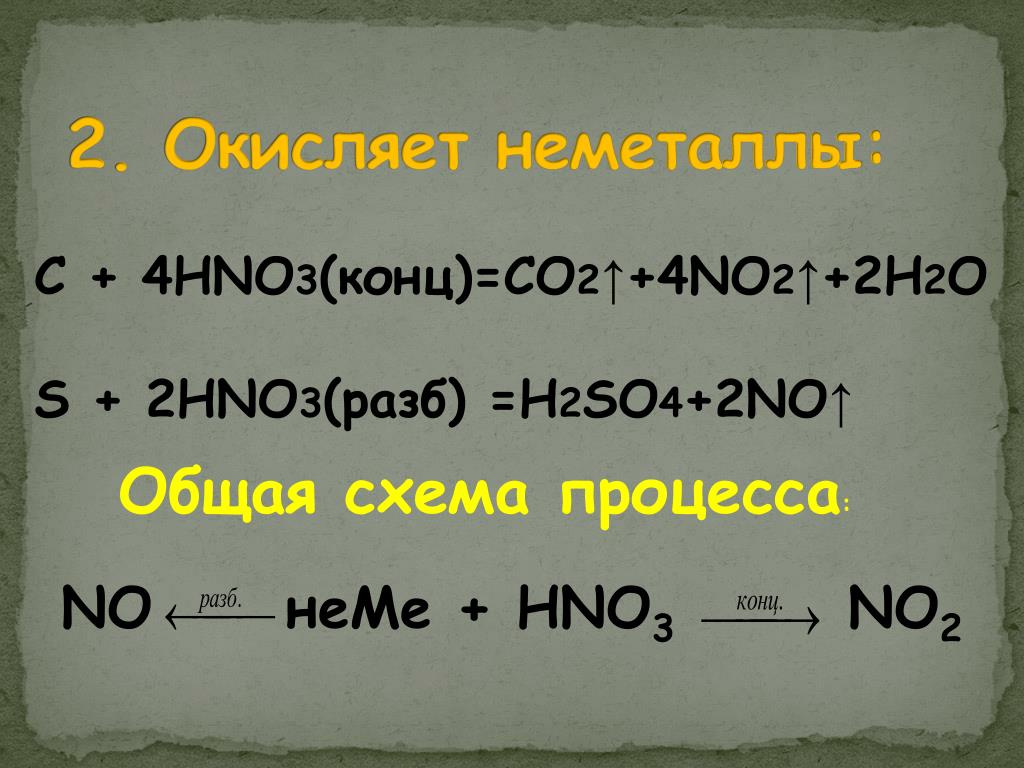
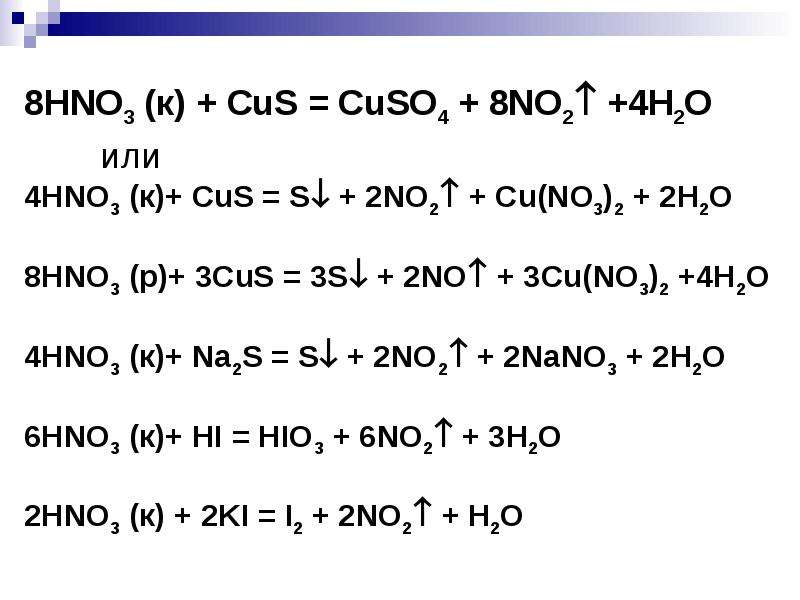
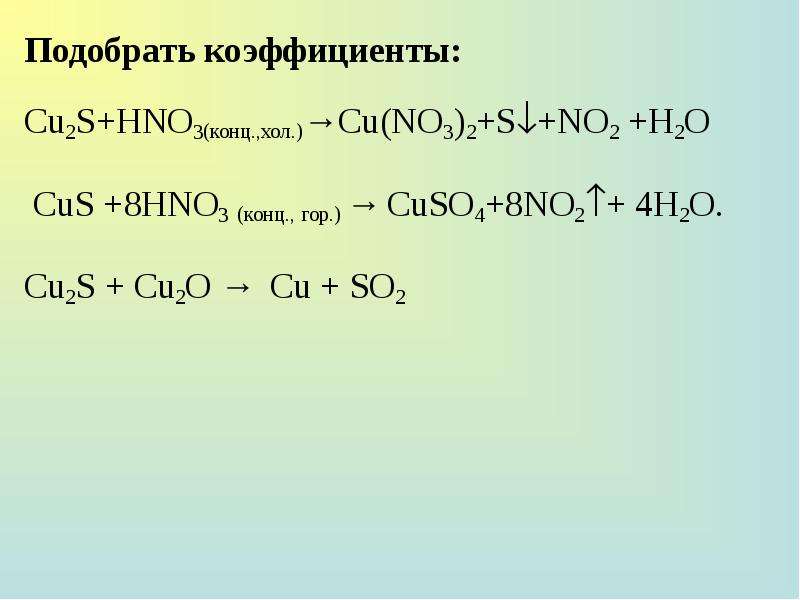
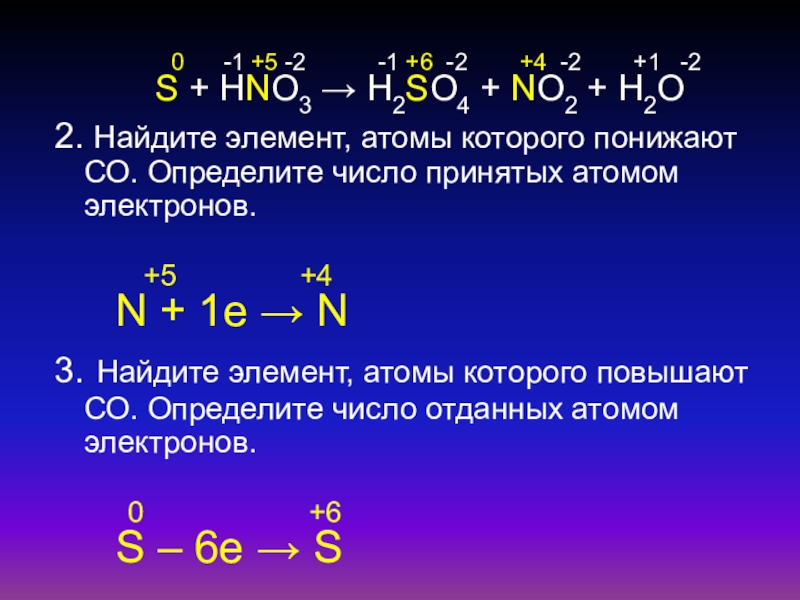 Кислотами-окислителями являются кислоты: H 2 SO 4 (конц.) и HNO 3 (любой концентрации). Fe + 2HCl → FeCl 2 + H2 (соль Fe +2, HCl любой концентрации). Cr + …
Кислотами-окислителями являются кислоты: H 2 SO 4 (конц.) и HNO 3 (любой концентрации). Fe + 2HCl → FeCl 2 + H2 (соль Fe +2, HCl любой концентрации). Cr + …
 To be balanced, every element in HNO3 + H2S = H2O + NO + S must have the same number of atoms on each side of the equation. When using the inspection method (also known as the …
To be balanced, every element in HNO3 + H2S = H2O + NO + S must have the same number of atoms on each side of the equation. When using the inspection method (also known as the …
Еще по теме:




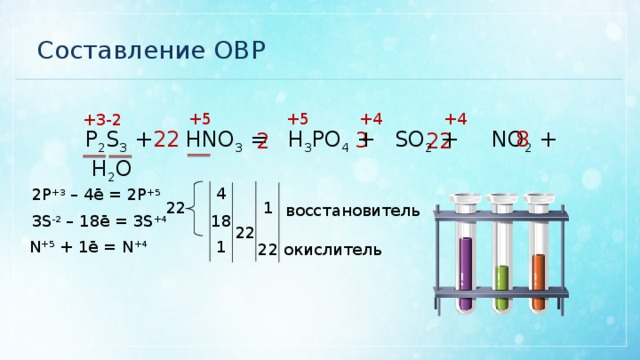


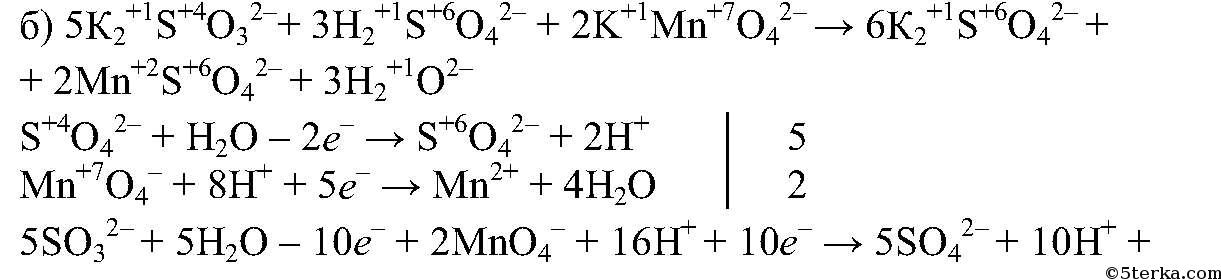

Еще по теме: