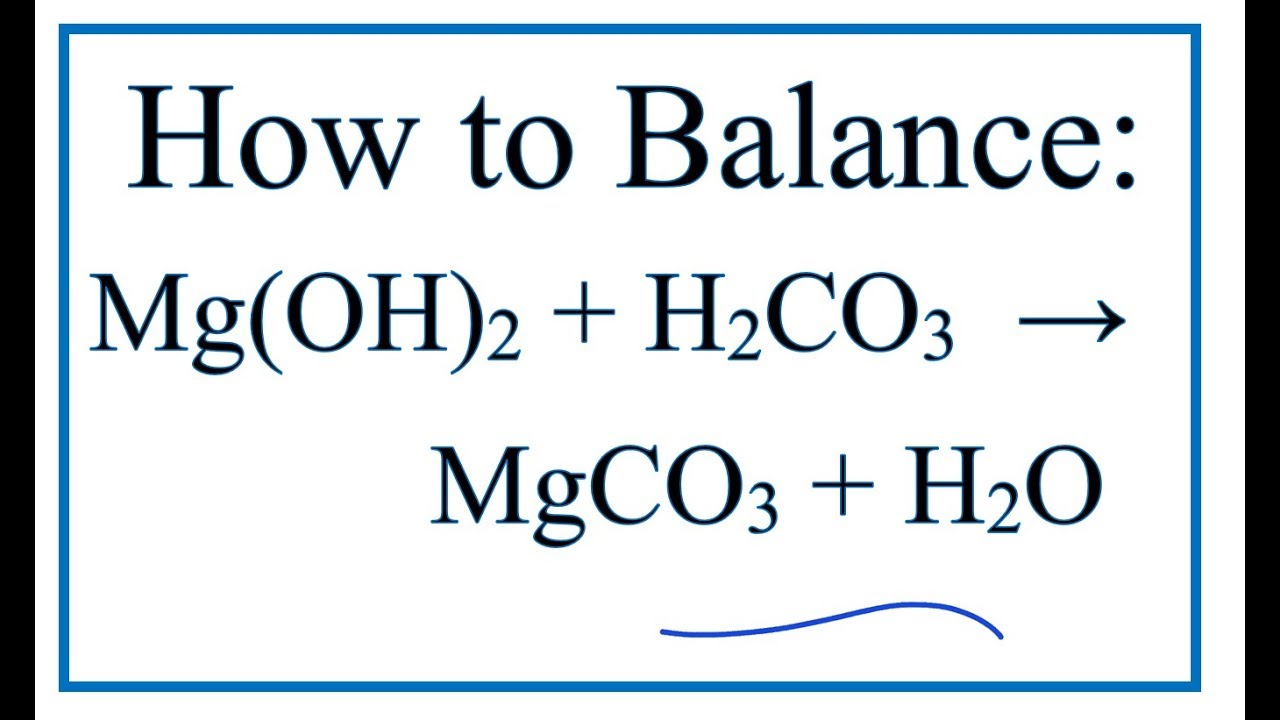
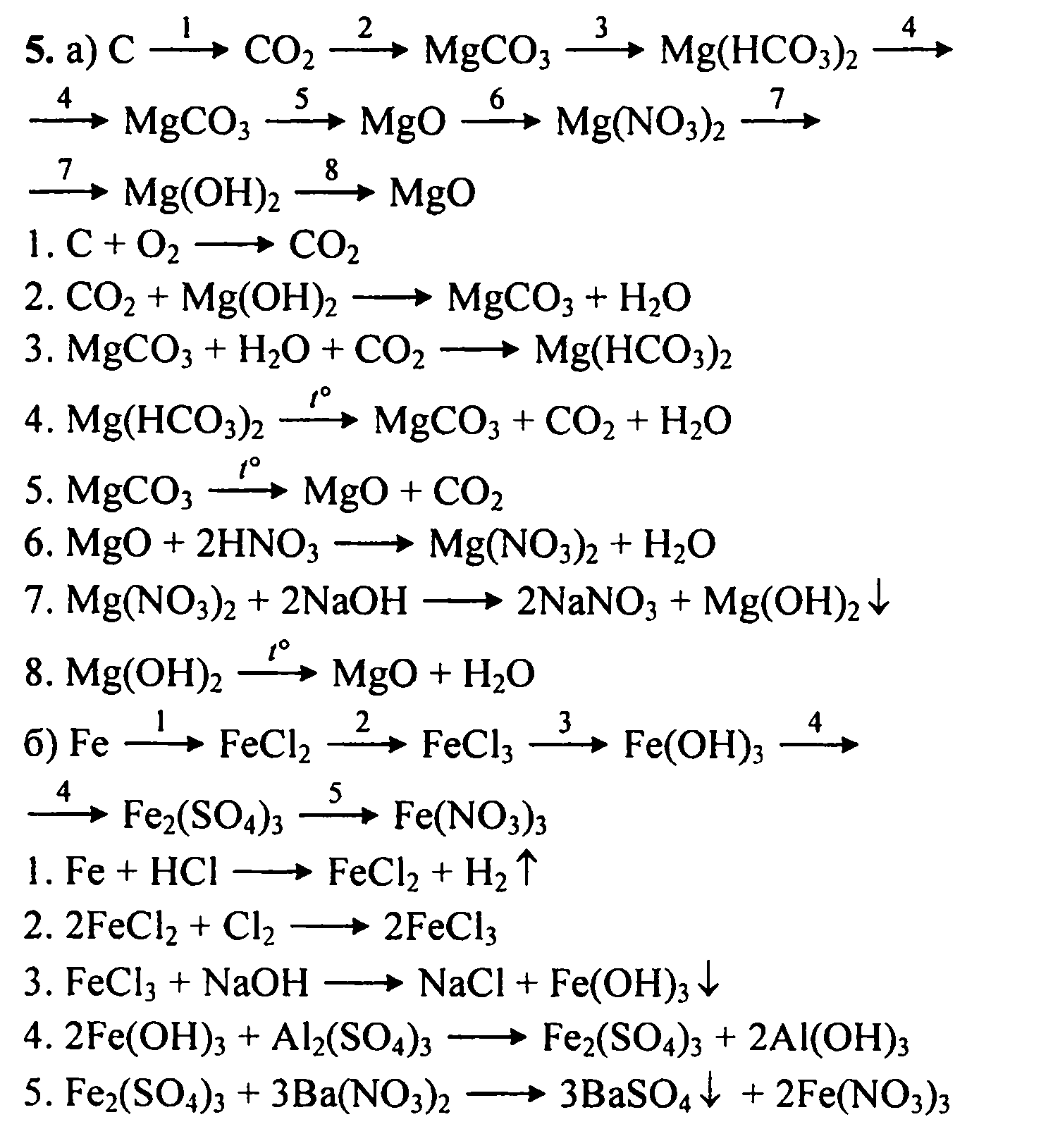
 Это кислотно-щелочная реакция (нейтрализа́ция): Mg(OH)2 является щелочным, CO2 представляет собой кислоту. Решенное и коэффициентами уравнение реакции Mg …
Это кислотно-щелочная реакция (нейтрализа́ция): Mg(OH)2 является щелочным, CO2 представляет собой кислоту. Решенное и коэффициентами уравнение реакции Mg …
 CO2 + Mg(OH)2 = Mg2(CO3)(OH)2 + H2O is a Double Displacement (Metathesis) reaction where one mole of Carbon Dioxide [CO 2] and two moles of Magnesium Hydroxide [Mg(OH) 2] react …
CO2 + Mg(OH)2 = Mg2(CO3)(OH)2 + H2O is a Double Displacement (Metathesis) reaction where one mole of Carbon Dioxide [CO 2] and two moles of Magnesium Hydroxide [Mg(OH) 2] react …
 1 mg(oh) 2 + 2 co 2 = 1 mg + 2 hco 3 O is balanced: 6 atoms in reagents and 6 atoms in products. All atoms are now balanced and the whole equation is fully balanced:
1 mg(oh) 2 + 2 co 2 = 1 mg + 2 hco 3 O is balanced: 6 atoms in reagents and 6 atoms in products. All atoms are now balanced and the whole equation is fully balanced:
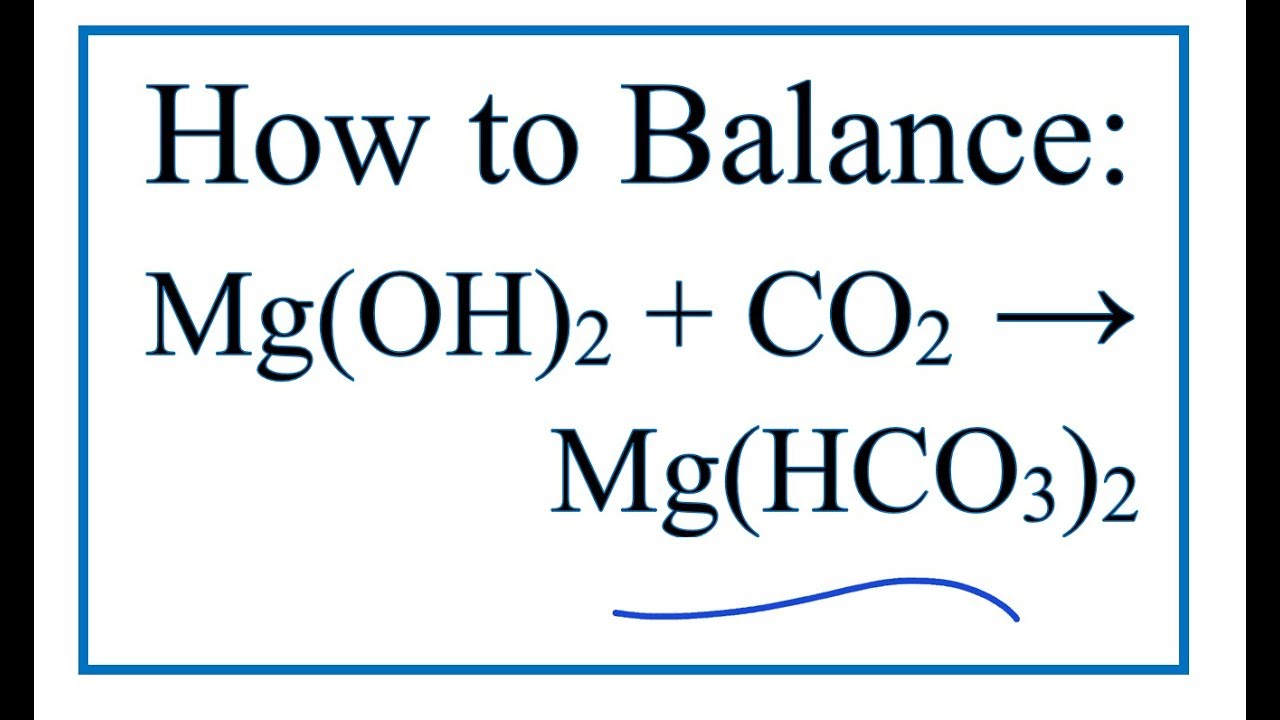
 In this video we'll balance the equation Mg (OH)2 + CO2 = Mg (HCO3)2 and provide the correct coefficients for each compound..more. To balance Mg (OH)2 + CO2 = Mg (HCO3)2 you'll need to.
In this video we'll balance the equation Mg (OH)2 + CO2 = Mg (HCO3)2 and provide the correct coefficients for each compound..more. To balance Mg (OH)2 + CO2 = Mg (HCO3)2 you'll need to.
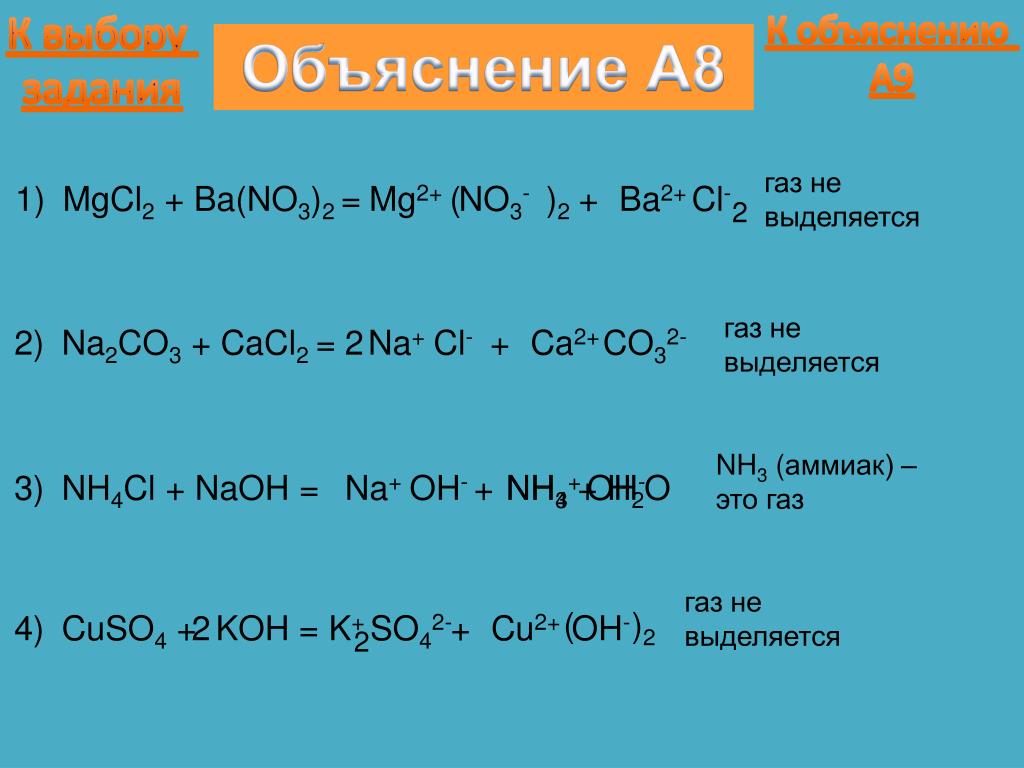
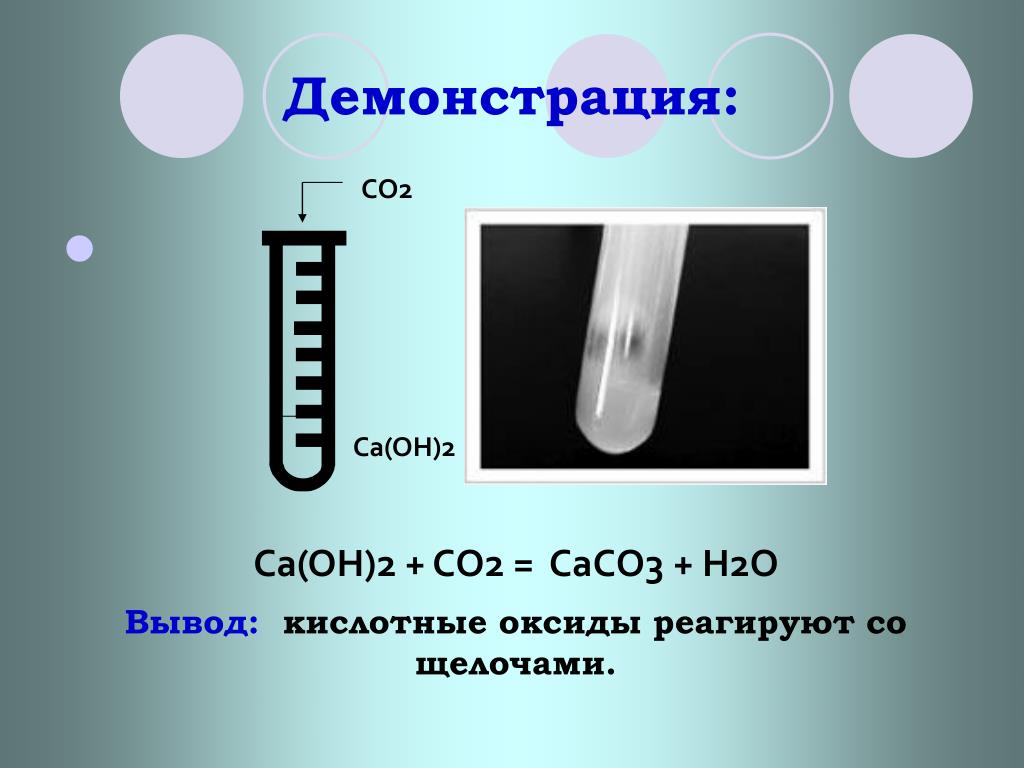
 Уравнение реакции взаимодействия гидроксида магния с оксидом углерода (IV). Получение карбоната магния и воды из гидроксида магния и оксида углерода (IV).
Уравнение реакции взаимодействия гидроксида магния с оксидом углерода (IV). Получение карбоната магния и воды из гидроксида магния и оксида углерода (IV).
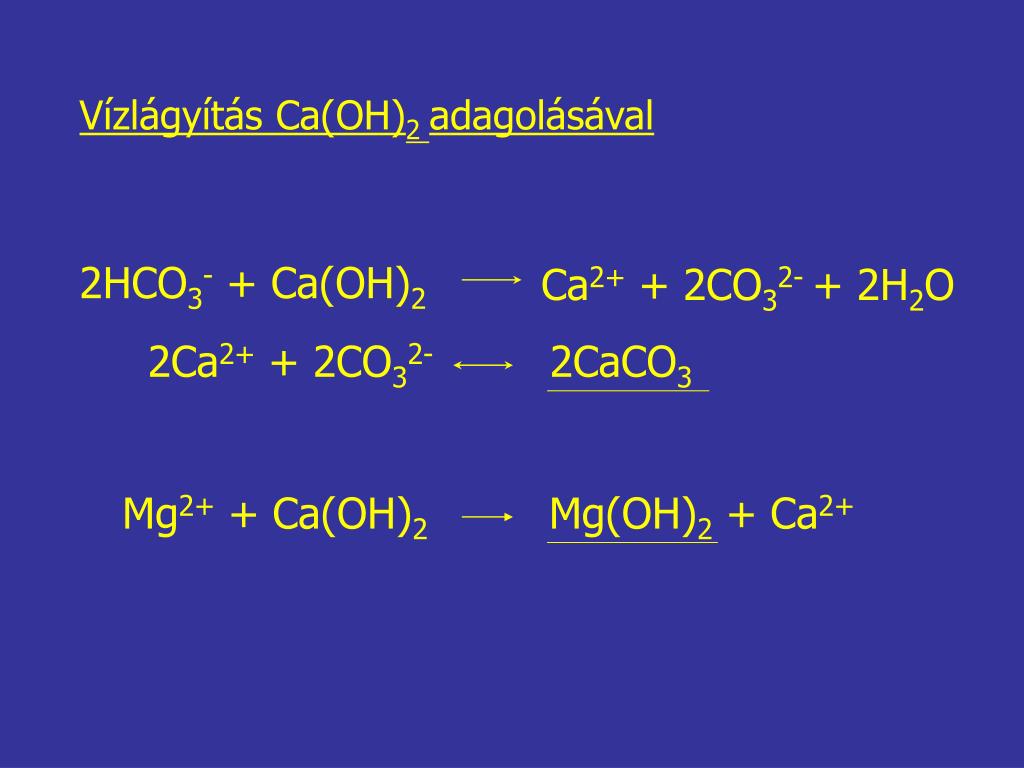 Magnesium bicarbonate exists only in aqueous solution. Magnesium does not form solid bicarbonate as like Lithium. To produce it, a suspension of magnesium hydroxide is …
Magnesium bicarbonate exists only in aqueous solution. Magnesium does not form solid bicarbonate as like Lithium. To produce it, a suspension of magnesium hydroxide is …
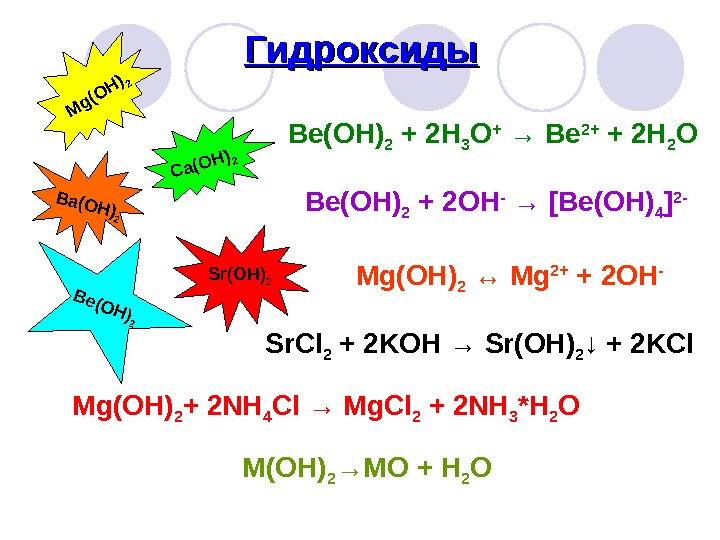
 Magnesium hydroxide react with carbon dioxide to produce magnesium dihydroxide-carbonate and water. Magnesium hydroxide - solid. The reaction proceeds at …
Magnesium hydroxide react with carbon dioxide to produce magnesium dihydroxide-carbonate and water. Magnesium hydroxide - solid. The reaction proceeds at …
 One of the most promising materials for carbon mineralization is Mg (OH) 2 which is highly reactive and capable of forming stable carbonates. Here we show a novel low-carbon …
One of the most promising materials for carbon mineralization is Mg (OH) 2 which is highly reactive and capable of forming stable carbonates. Here we show a novel low-carbon …
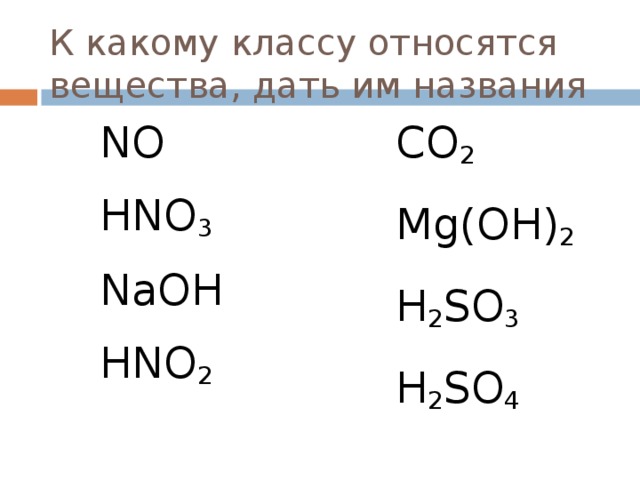
 Construct the rate of reaction expression for: CO_2 + Mg(OH)_2 H_2O + MgCO_3 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for each chemical species.
Construct the rate of reaction expression for: CO_2 + Mg(OH)_2 H_2O + MgCO_3 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for each chemical species.
 Решенное и коэффициентами уравнение реакции 2 CO2 + Mg (OH)2 → Mg (HCO3)2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 CO2 + Mg (OH)2 → Mg (HCO3)2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
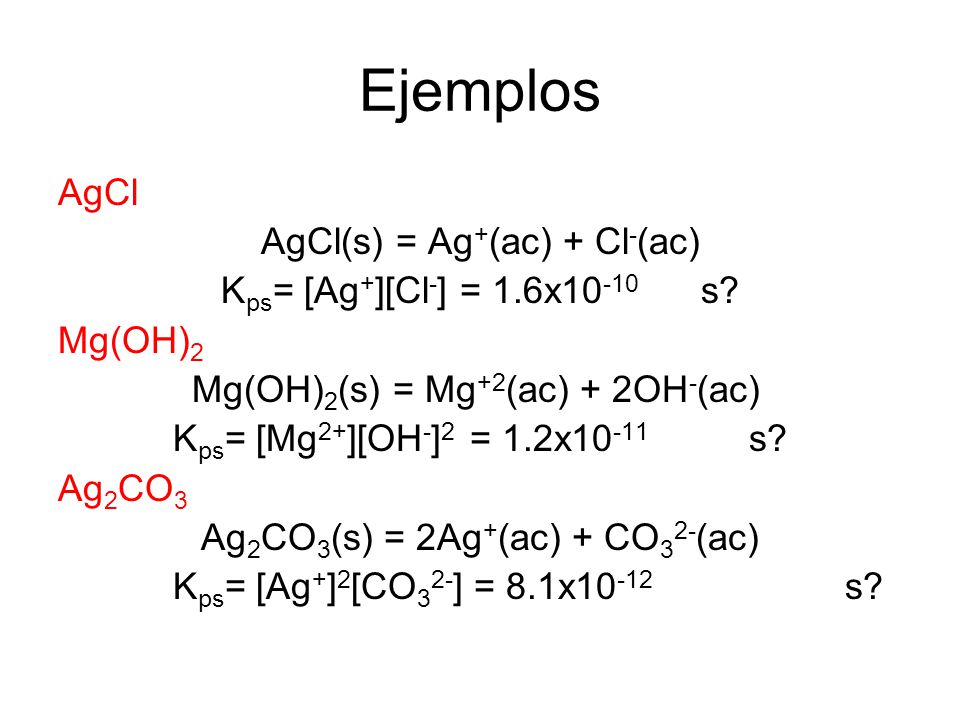
 From the solubility data, Mg(OH)2 is by far less soluble than MgCO3. From the Ksp data, any way you look at them, the solubility product of Mg(OH)2 is smaller than for MgCO3, indicating a …
From the solubility data, Mg(OH)2 is by far less soluble than MgCO3. From the Ksp data, any way you look at them, the solubility product of Mg(OH)2 is smaller than for MgCO3, indicating a …
 We found (1) H2O mols. significantly facilitate CO2 capture on MgO but not on Mg(OH)2, (2) formation of dehydration defects on Mg(OH)2 dramatically increases the CO2 …
We found (1) H2O mols. significantly facilitate CO2 capture on MgO but not on Mg(OH)2, (2) formation of dehydration defects on Mg(OH)2 dramatically increases the CO2 …
 To avoid dangerous climate change, new technologies must remove billions of tonnes of CO 2 from the atmosphere every year by mid-century. Here we detail a land-based …
To avoid dangerous climate change, new technologies must remove billions of tonnes of CO 2 from the atmosphere every year by mid-century. Here we detail a land-based …
 Recent results showed that Mg (OH) 2 carbonation in the slurry occurs spontaneously (Fricker and Park, 2013). In our study, Mg (OH) 2 is used as the absorbent due …
Recent results showed that Mg (OH) 2 carbonation in the slurry occurs spontaneously (Fricker and Park, 2013). In our study, Mg (OH) 2 is used as the absorbent due …
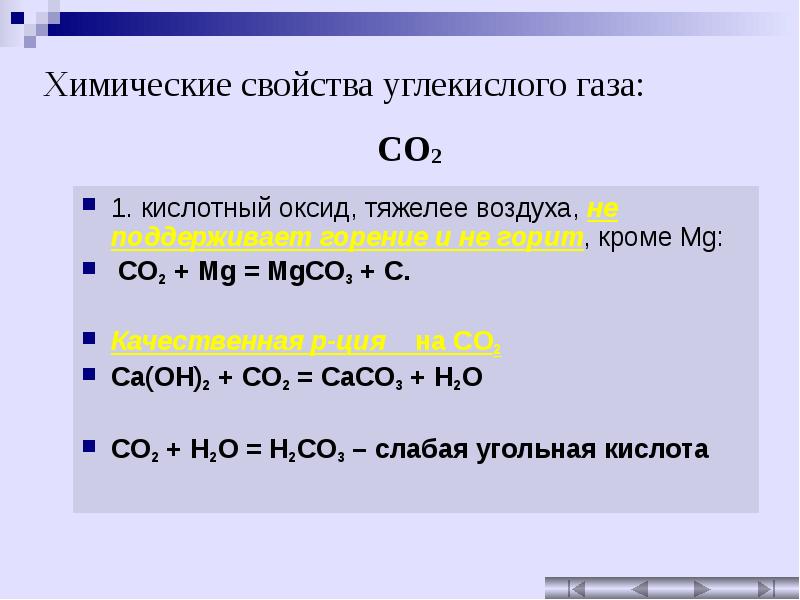 Решенное и коэффициентами уравнение реакции CO2 + Mg (OH)2 → H2O + MgCO3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции CO2 + Mg (OH)2 → H2O + MgCO3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Mechanistic investigations employing in situ Raman and attenuated total reflection surface-enhanced infrared absorption spectroscopy unravel that Mg (OH) 2 plays a pivotal role …
Mechanistic investigations employing in situ Raman and attenuated total reflection surface-enhanced infrared absorption spectroscopy unravel that Mg (OH) 2 plays a pivotal role …
Еще по теме:








Еще по теме: