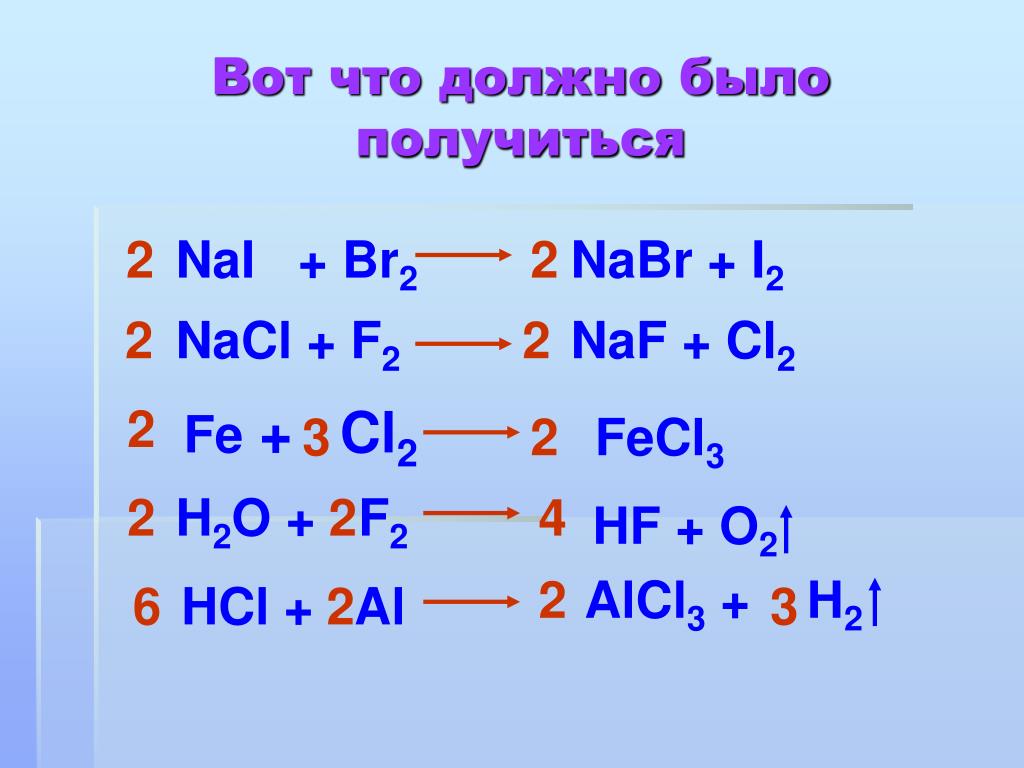
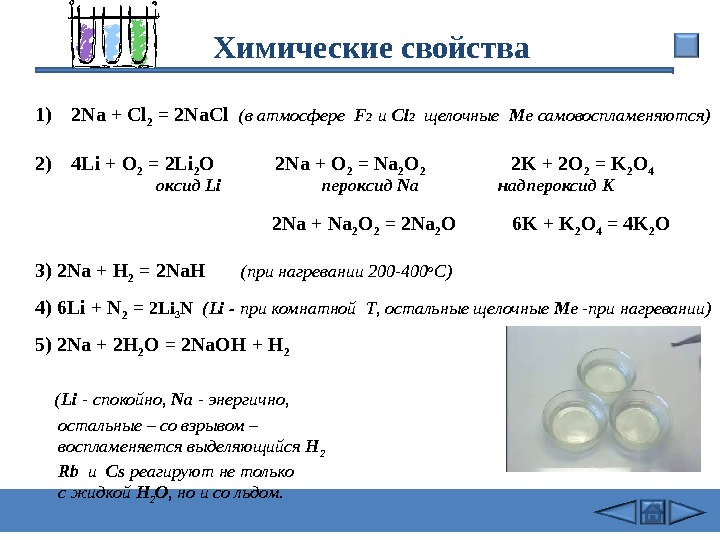
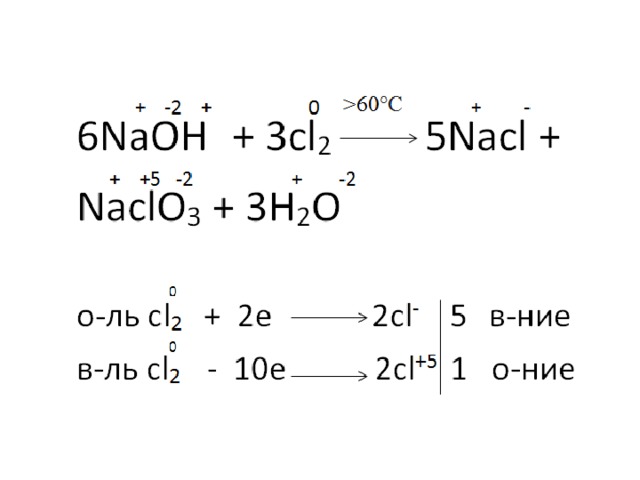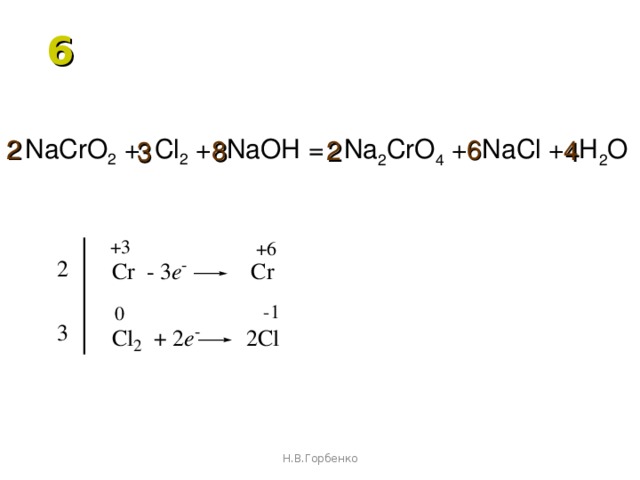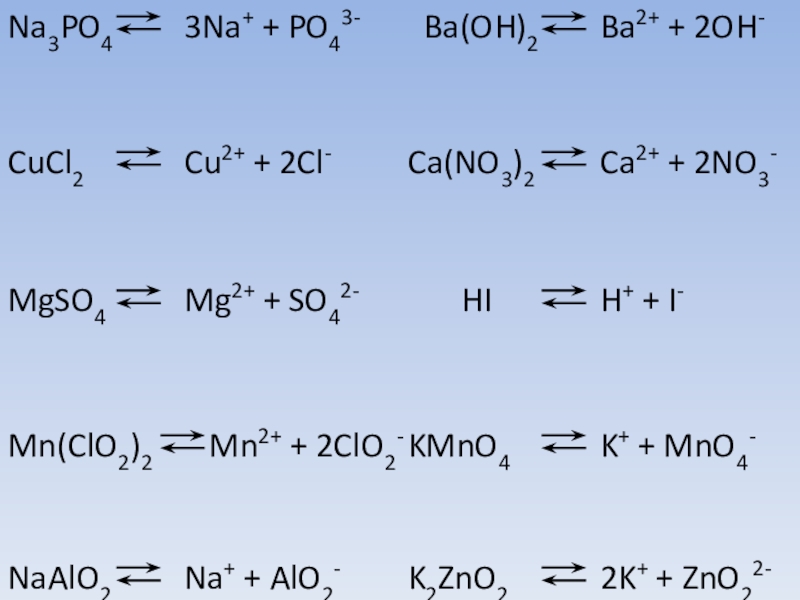Решенное и коэффициентами уравнение реакции 2 Na + Cl2 → 2 NaCl с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 Na + Cl2 → 2 NaCl с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 1 Na + 1 Cl 2 = 1 NaCl For each element, we check if the number of atoms is balanced on both sides of the equation. Na is balanced: 1 atom in reagents and 1 atom in products.
1 Na + 1 Cl 2 = 1 NaCl For each element, we check if the number of atoms is balanced on both sides of the equation. Na is balanced: 1 atom in reagents and 1 atom in products.
 Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music….
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music….

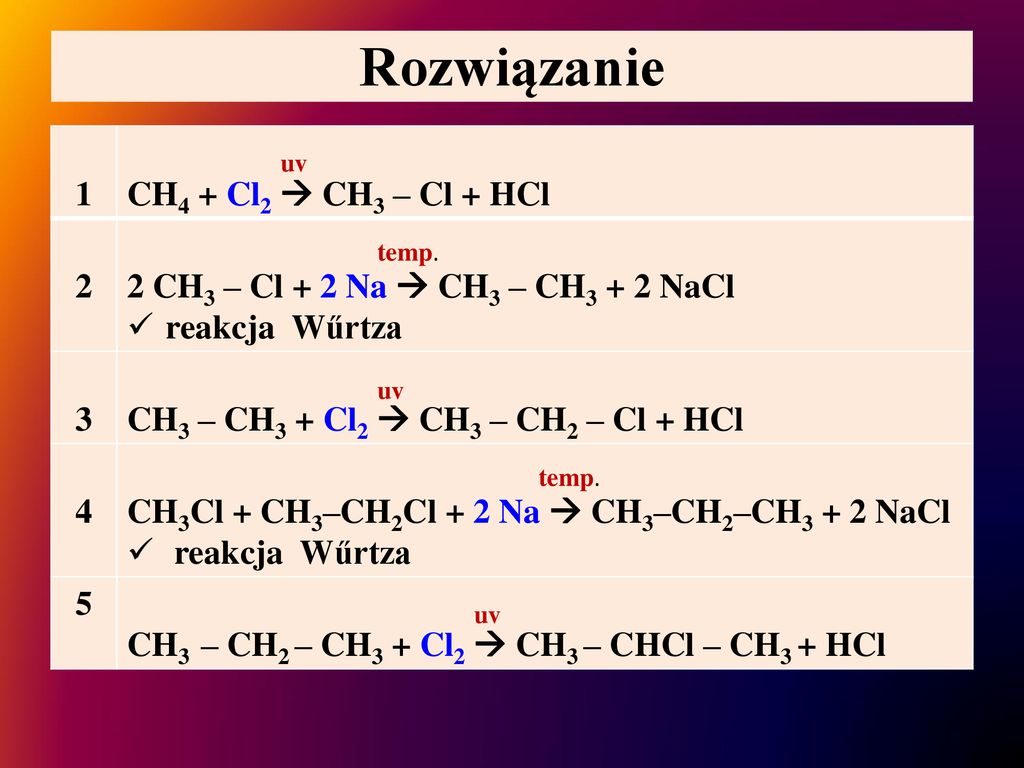
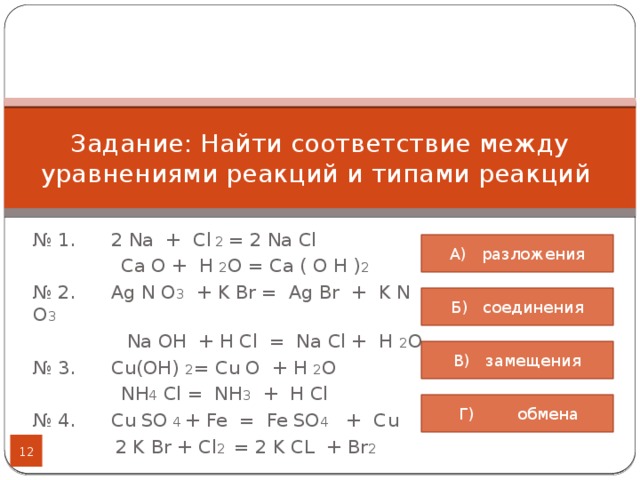
 Na – натрий Cl 2 – хлор. Продукты NaCl – хлорид натрия
Na – натрий Cl 2 – хлор. Продукты NaCl – хлорид натрия
 20,842 views • Apr 21, 2023 • Balancing Chemical Equations with Dr. B. Explanation of how to balance the equation Na + Cl2 = NaCl. Part of a Short Course on Balancing Equations Playlist: • A.
20,842 views • Apr 21, 2023 • Balancing Chemical Equations with Dr. B. Explanation of how to balance the equation Na + Cl2 = NaCl. Part of a Short Course on Balancing Equations Playlist: • A.
 How to Balance: Na + Cl2 = NaCl. This is a classic chemical equation used to demonstrate balancing. Visit https://www.Breslyn.org for video guides on balancing equations and more!In order.
How to Balance: Na + Cl2 = NaCl. This is a classic chemical equation used to demonstrate balancing. Visit https://www.Breslyn.org for video guides on balancing equations and more!In order.
 Na+Cl2. en. Related Symbolab blog posts. Practice Makes Perfect. Learning math takes practice, lots of practice. Just like running, it takes practice and dedication. If you want. Enter a problem. Cooking Calculators. Cooking Measurement Converter Cooking Ingredient Converter Cake Pan Converter More calculators.
Na+Cl2. en. Related Symbolab blog posts. Practice Makes Perfect. Learning math takes practice, lots of practice. Just like running, it takes practice and dedication. If you want. Enter a problem. Cooking Calculators. Cooking Measurement Converter Cooking Ingredient Converter Cake Pan Converter More calculators.
 Phản ứng Na + Cl 2 hay Na ra NaCl hoặc Cl 2 ra NaCl thuộc loại phản ứng oxi hóa khử, phản ứng hóa hợp đã được cân bằng chính xác và chi tiết nhất. Bên cạnh đó là một số bài tập có liên quan về Na có lời giải, mời các bạn đón xem: 2Na + Cl 2 → 2NaCl. Điều kiện phản ứng. - Nhiệt độ. Cách thực hiện phản ứng.
Phản ứng Na + Cl 2 hay Na ra NaCl hoặc Cl 2 ra NaCl thuộc loại phản ứng oxi hóa khử, phản ứng hóa hợp đã được cân bằng chính xác và chi tiết nhất. Bên cạnh đó là một số bài tập có liên quan về Na có lời giải, mời các bạn đón xem: 2Na + Cl 2 → 2NaCl. Điều kiện phản ứng. - Nhiệt độ. Cách thực hiện phản ứng.
 Ответы 1. Cl2 + Na OH = NaCl + NaClO + H20. Расставим степени окисления всех элементов: Cl^ (0)2 + Na ^ (+1) O^ (-2) H^ (+1) = Na^ (+1) Cl ^ (+1) + Na^ (+1) Cl^ (+1) O^ (-2) + H^ (+1)20^ (-2) Определим элементы, изменившие степени окисления: это хлор .
Ответы 1. Cl2 + Na OH = NaCl + NaClO + H20. Расставим степени окисления всех элементов: Cl^ (0)2 + Na ^ (+1) O^ (-2) H^ (+1) = Na^ (+1) Cl ^ (+1) + Na^ (+1) Cl^ (+1) O^ (-2) + H^ (+1)20^ (-2) Определим элементы, изменившие степени окисления: это хлор .
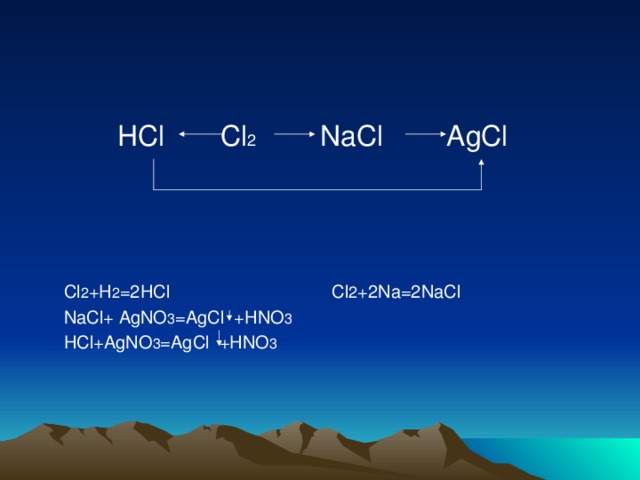
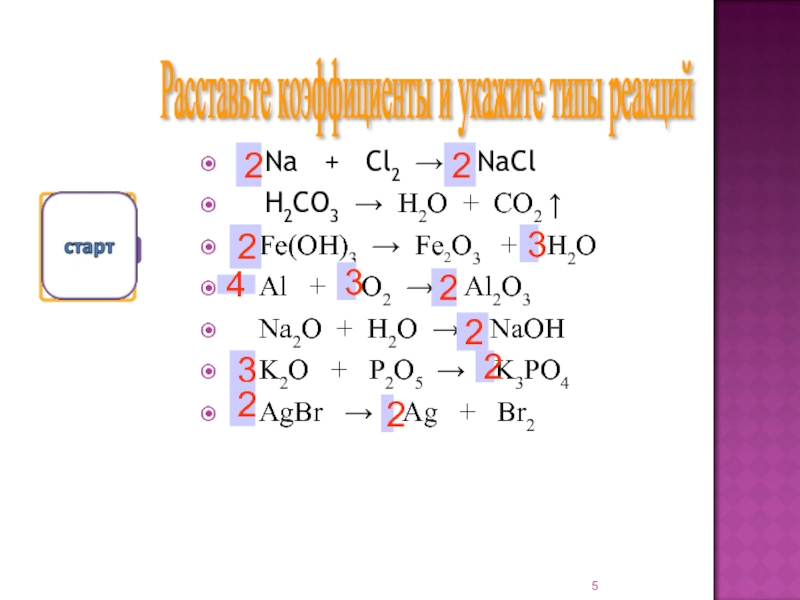
 Cl2 + Na = ClNa is a Synthesis reaction where one mole of Dichlorine [Cl 2] and two moles of Sodium [Na] combine to form two moles of Sodium Chloride [ClNa]
Cl2 + Na = ClNa is a Synthesis reaction where one mole of Dichlorine [Cl 2] and two moles of Sodium [Na] combine to form two moles of Sodium Chloride [ClNa]
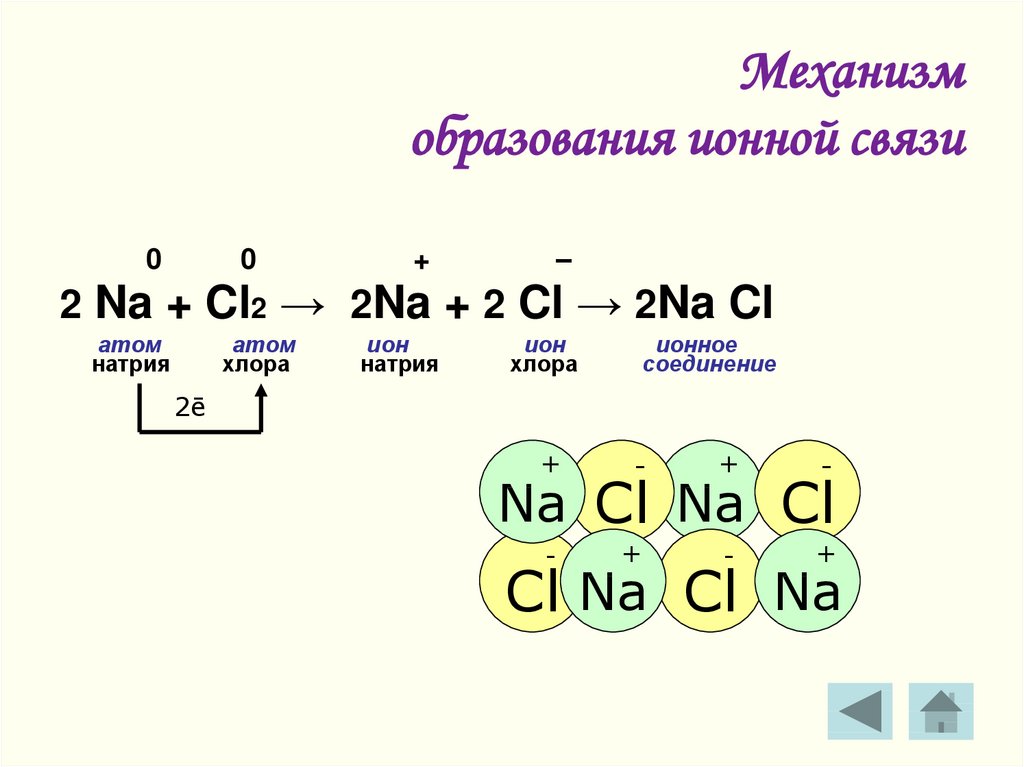 Решенное и коэффициентами уравнение реакции Cl2 + 2 Na → 2 NaCl с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции Cl2 + 2 Na → 2 NaCl с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
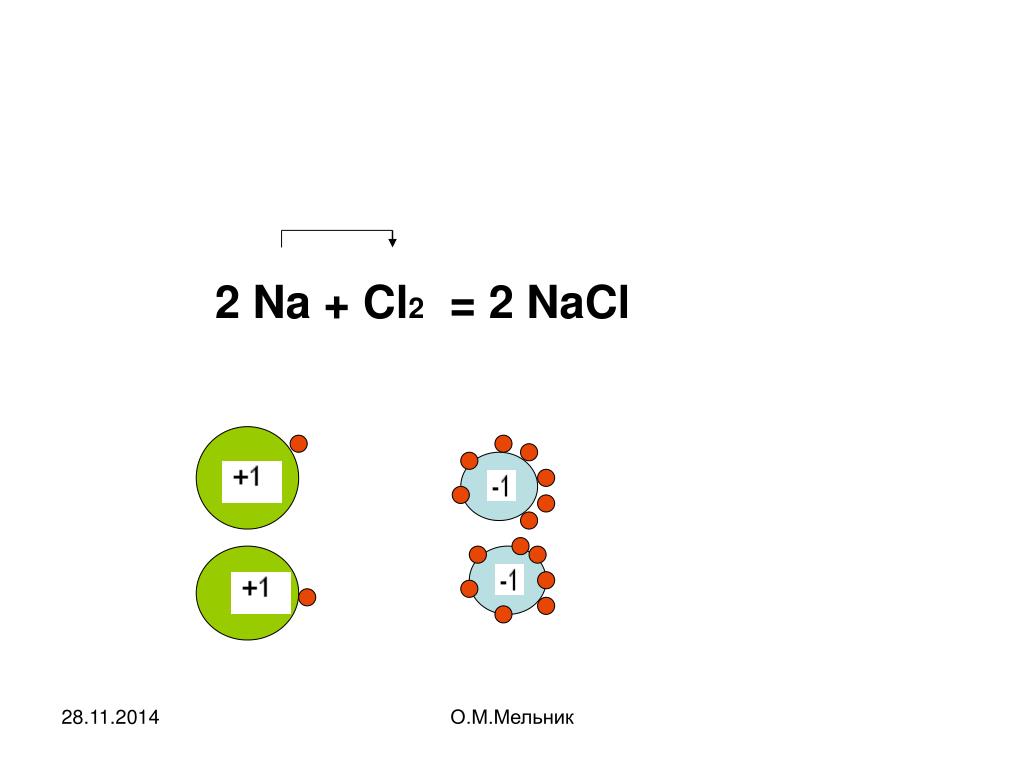
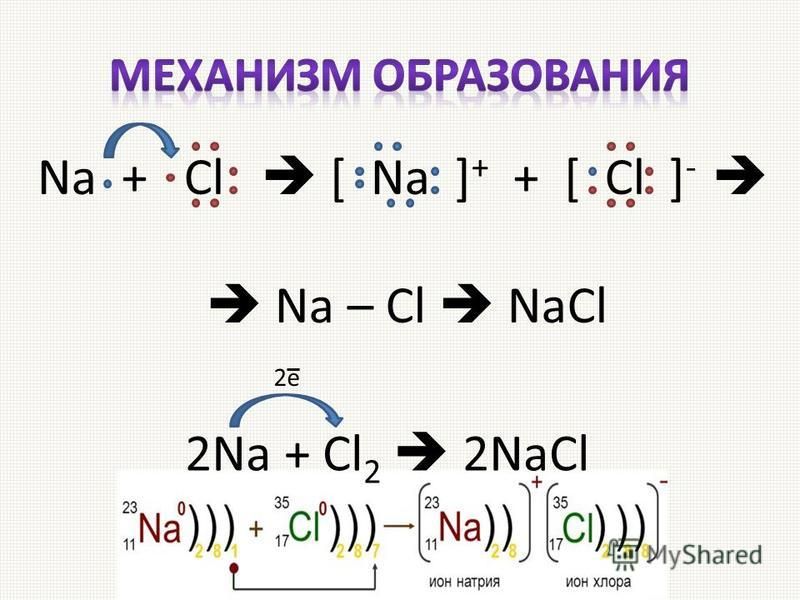
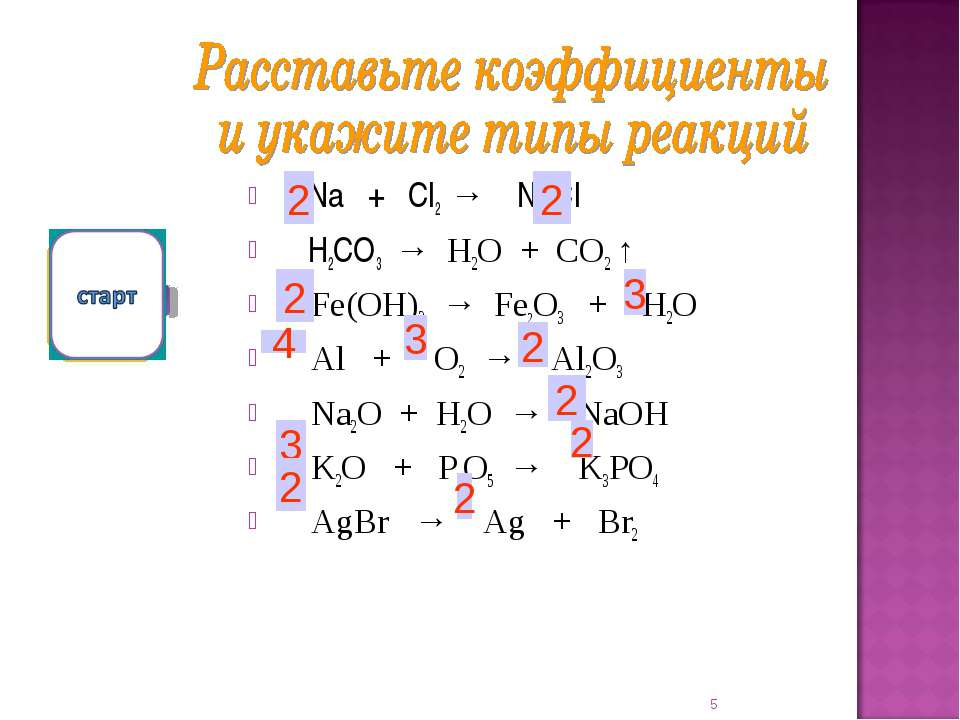 Ответы 1. Na- металлическая (образуется между атомами металла); Cl2 - ковалентная неполярная (между атомами двух одинаковых неметаллом); NaCl - ионная (образуется между атомами металла и неметалла.
Ответы 1. Na- металлическая (образуется между атомами металла); Cl2 - ковалентная неполярная (между атомами двух одинаковых неметаллом); NaCl - ионная (образуется между атомами металла и неметалла.
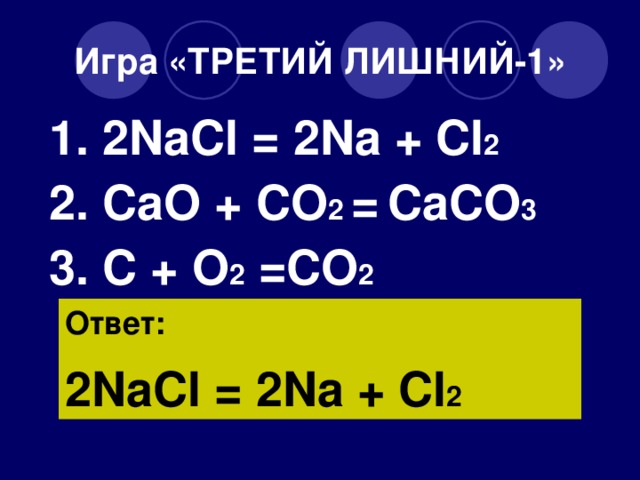 1 NaCl 2 = 1 Na + 1 Cl 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Na is balanced: 1 atom in reagents and 1 atom in products.
1 NaCl 2 = 1 Na + 1 Cl 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Na is balanced: 1 atom in reagents and 1 atom in products.
 Решенное и коэффициентами уравнение реакции Cl2 + 2 NaOH → H2O + NaCl + NaClO с дополненными продуктами.
Решенное и коэффициентами уравнение реакции Cl2 + 2 NaOH → H2O + NaCl + NaClO с дополненными продуктами.
 1 Cl 2 + 1 Na = 1 NaCl For each element, we check if the number of atoms is balanced on both sides of the equation. Cl is not balanced: 2 atoms in reagents and 1 atom in products.
1 Cl 2 + 1 Na = 1 NaCl For each element, we check if the number of atoms is balanced on both sides of the equation. Cl is not balanced: 2 atoms in reagents and 1 atom in products.
Еще по теме:











Еще по теме: