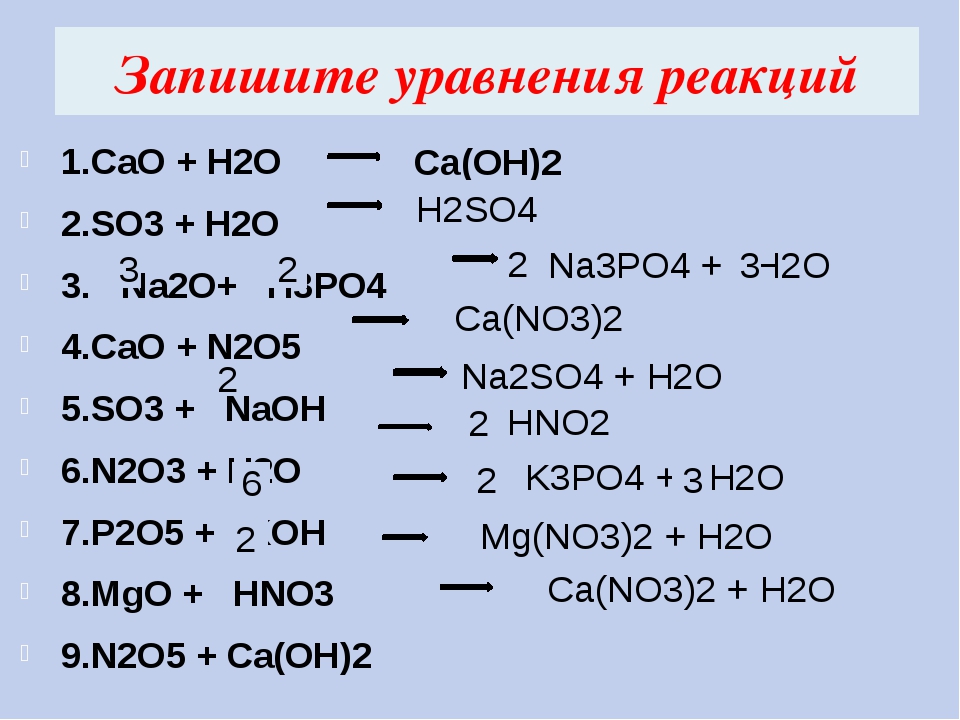
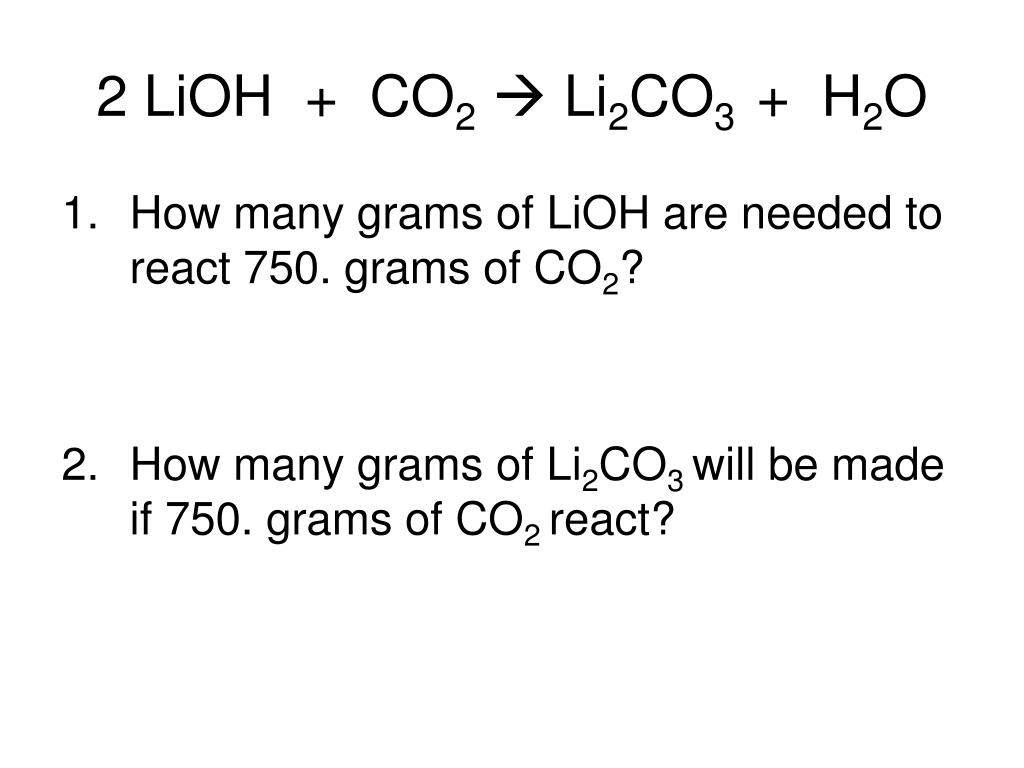Это кислотно-щелочная реакция (нейтрализа́ция): Li2O является щелочным, H3PO4 представляет собой кислоту. Решенное и коэффициентами уравнение реакции 3 Li2O + …
Это кислотно-щелочная реакция (нейтрализа́ция): Li2O является щелочным, H3PO4 представляет собой кислоту. Решенное и коэффициентами уравнение реакции 3 Li2O + …
 Li2O + H3PO4 = Li3PO4 + H2O is a Double Displacement (Metathesis) reaction where three moles of Lithium Oxide [Li 2 O] and two moles of Phosphoric Acid [H 3 PO 4] react to form two …
Li2O + H3PO4 = Li3PO4 + H2O is a Double Displacement (Metathesis) reaction where three moles of Lithium Oxide [Li 2 O] and two moles of Phosphoric Acid [H 3 PO 4] react to form two …
 Li2O + H3PO4 = H2O + Li3PO4 is a Double Displacement (Metathesis) reaction where three moles of Lithium Oxide [Li 2 O] and two moles of Phosphoric Acid [H 3 PO 4] react to form …
Li2O + H3PO4 = H2O + Li3PO4 is a Double Displacement (Metathesis) reaction where three moles of Lithium Oxide [Li 2 O] and two moles of Phosphoric Acid [H 3 PO 4] react to form …
 This is an acid-base reaction ( neutralization ): Li2O is a base, H3PO4 is an acid. Appearance: Hygroscopic colourless crystals ; Thick, colorless, odorless, crystalline solid. [Note: Often used …
This is an acid-base reaction ( neutralization ): Li2O is a base, H3PO4 is an acid. Appearance: Hygroscopic colourless crystals ; Thick, colorless, odorless, crystalline solid. [Note: Often used …
 Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
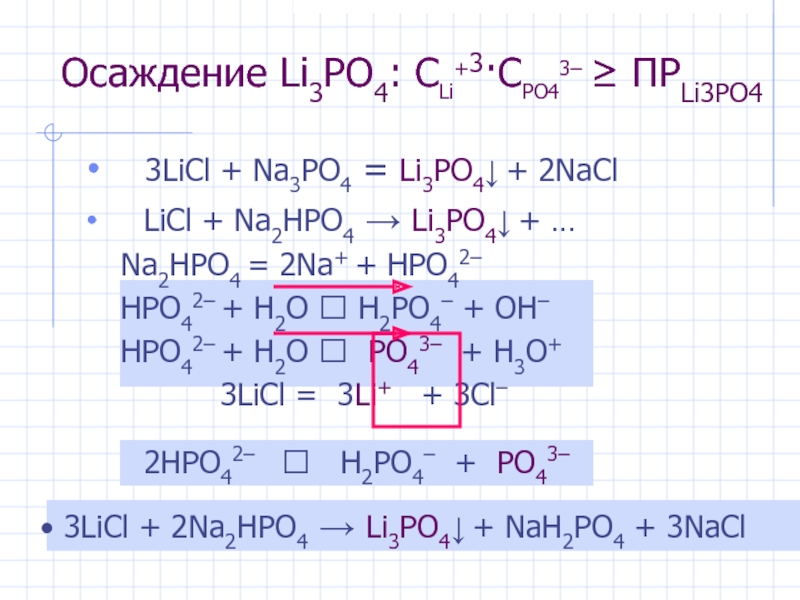 Li3PO4 + H2O = Li2O + H3PO4 is a Double Displacement (Metathesis) reaction where two moles of Lithium Phosphate [Li 3 PO 4] and three moles of Water [H 2 O] react to form three moles of …
Li3PO4 + H2O = Li2O + H3PO4 is a Double Displacement (Metathesis) reaction where two moles of Lithium Phosphate [Li 3 PO 4] and three moles of Water [H 2 O] react to form three moles of …
 Construct the equilibrium constant, K, expression for: H_3PO_4 + Li_2O H_2O + Li_3PO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the …
Construct the equilibrium constant, K, expression for: H_3PO_4 + Li_2O H_2O + Li_3PO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the …
 Решенное и коэффициентами уравнение реакции 2 h3po4 + 3 li2o → 3 h2o + 2 li3po4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 h3po4 + 3 li2o → 3 h2o + 2 li3po4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Units: molar mass - g/mol, weight - g. Please tell about this free chemistry software to your friends! Direct link to this balanced equation: Instructions on balancing chemical equations:
Units: molar mass - g/mol, weight - g. Please tell about this free chemistry software to your friends! Direct link to this balanced equation: Instructions on balancing chemical equations:
 Solved and balanced chemical equation 2 H3PO4 + 3 Li2O → 3 H2O + 2 Li3PO4 with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation 2 H3PO4 + 3 Li2O → 3 H2O + 2 Li3PO4 with completed products. Application for completing products and balancing equations.
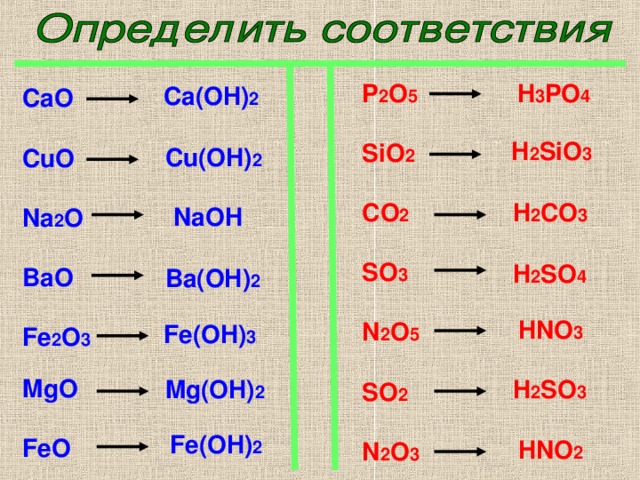
 3) Al 2 O 3 + 3H 2 SO 4 = Al 2 (SO 4) 3 + 3H 2 O (при взаимодействии оксида алюминия с серной кислотой, образуется сульфат алюминия и вода); 4) NaCl + HCl = реакция не …
3) Al 2 O 3 + 3H 2 SO 4 = Al 2 (SO 4) 3 + 3H 2 O (при взаимодействии оксида алюминия с серной кислотой, образуется сульфат алюминия и вода); 4) NaCl + HCl = реакция не …
 H3PO4 + Li2O = Li3PO4 + H2O is a Double Displacement (Metathesis) reaction where two moles of Phosphoric Acid [H 3 PO 4] and three moles of Lithium Oxide [Li 2 O] react to form two …
H3PO4 + Li2O = Li3PO4 + H2O is a Double Displacement (Metathesis) reaction where two moles of Phosphoric Acid [H 3 PO 4] and three moles of Lithium Oxide [Li 2 O] react to form two …
 To be balanced, every element in Li2O + H3PO4 = Li2HPO4 + H2O must have the same number of atoms on each side of the equation. When using the inspection method (also known as the …
To be balanced, every element in Li2O + H3PO4 = Li2HPO4 + H2O must have the same number of atoms on each side of the equation. When using the inspection method (also known as the …
 Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
 Это кислотно-щелочная реакция ( нейтрализа́ция ): H3PO4 представляет собой кислоту, LiOH является щелочным. Решенное и коэффициентами уравнение реакции H3PO4 + 2 …
Это кислотно-щелочная реакция ( нейтрализа́ция ): H3PO4 представляет собой кислоту, LiOH является щелочным. Решенное и коэффициентами уравнение реакции H3PO4 + 2 …
 LiOH + H3PO4 = Li3PO4 + H2O is a Double Displacement (Metathesis) reaction where three moles of aqueous Lithium Hydroxide [LiOH] and one mole of aqueous Phosphoric Acid [H 3 …
LiOH + H3PO4 = Li3PO4 + H2O is a Double Displacement (Metathesis) reaction where three moles of aqueous Lithium Hydroxide [LiOH] and one mole of aqueous Phosphoric Acid [H 3 …
Еще по теме:












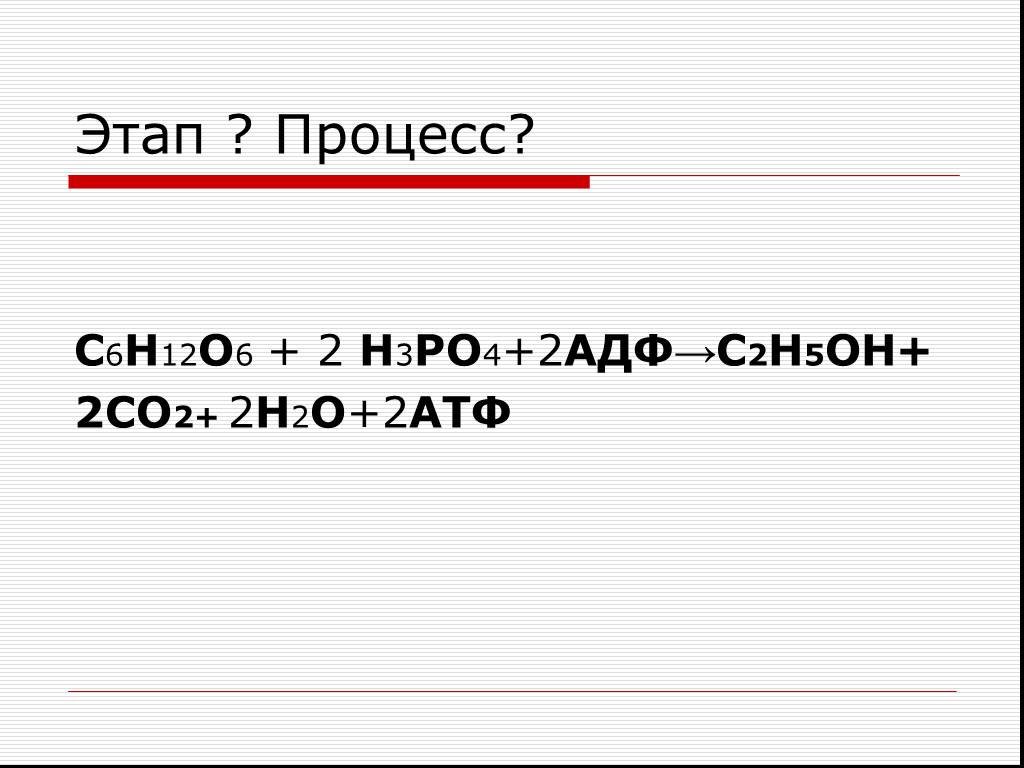
Еще по теме: