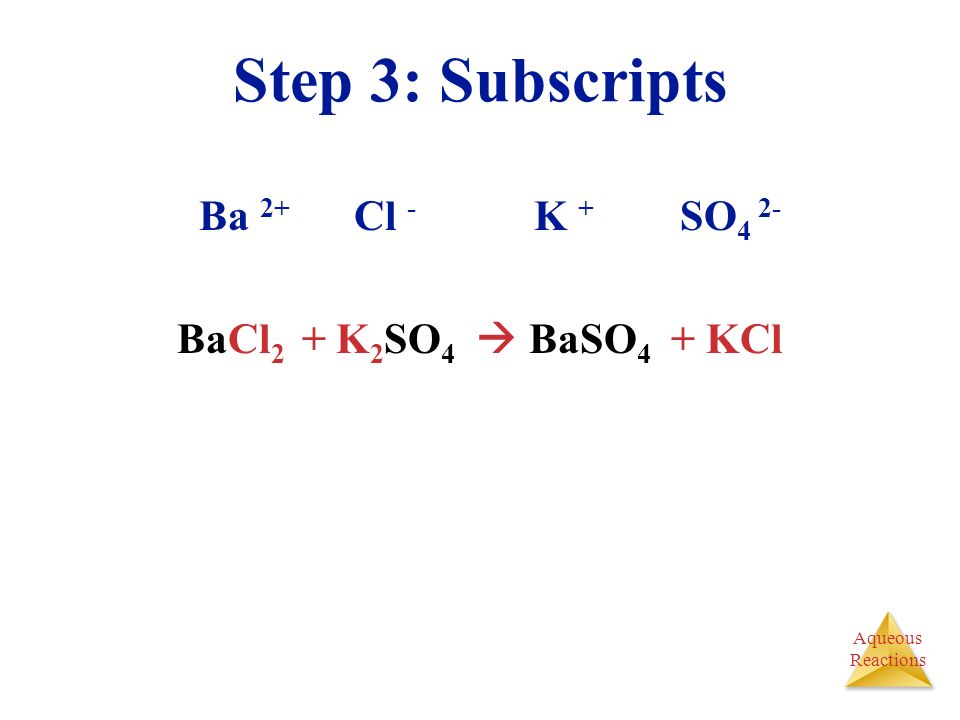
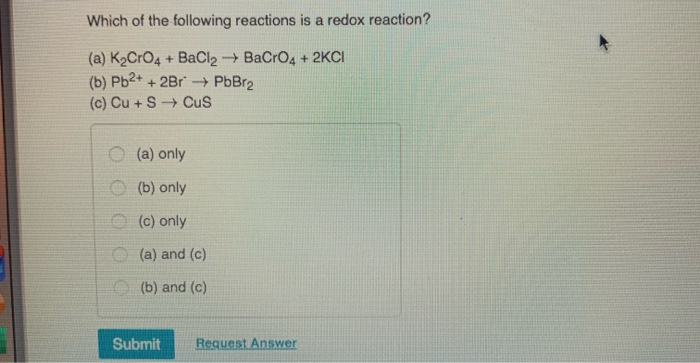
Решенное и коэффициентами уравнение реакции BaCl2 + K2CrO4 → BaCrO4 + 2 KCl с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции BaCl2 + K2CrO4 → BaCrO4 + 2 KCl с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
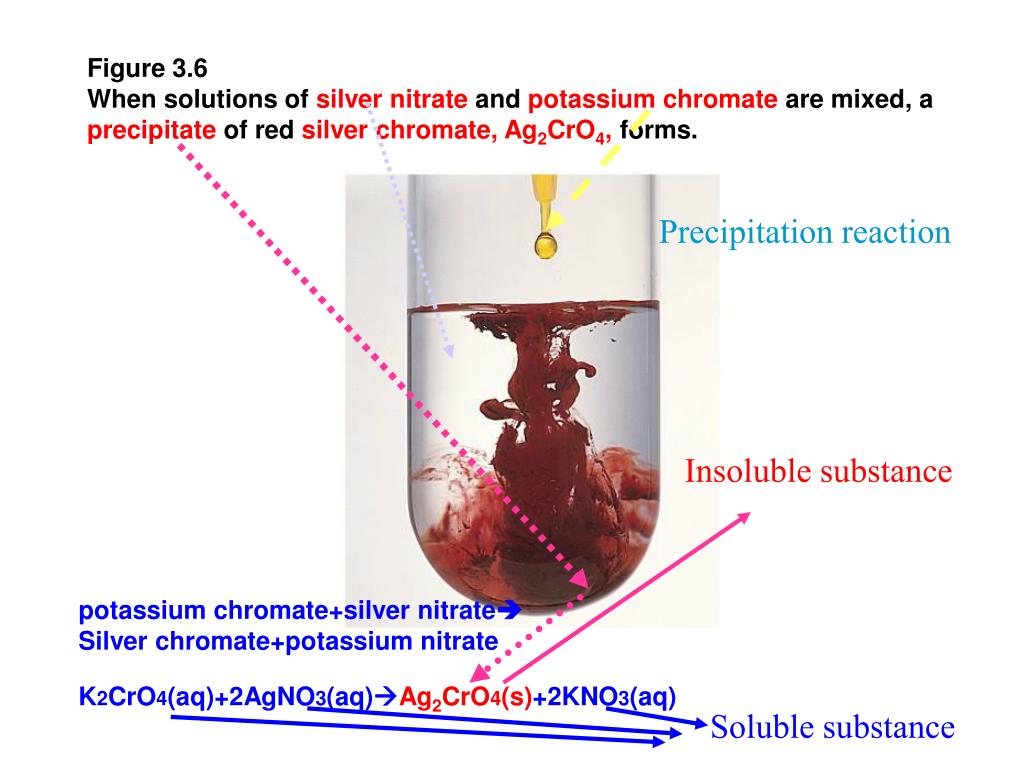
 K2CrO4(aq)+BaCl2(aq)→2KCl(aq)+BaCrO4(s) Это реакция осаждения: BaCrO4 сформированный осаждаться (преципитатом). Решенное и коэффициентами уравнение …
K2CrO4(aq)+BaCl2(aq)→2KCl(aq)+BaCrO4(s) Это реакция осаждения: BaCrO4 сформированный осаждаться (преципитатом). Решенное и коэффициентами уравнение …
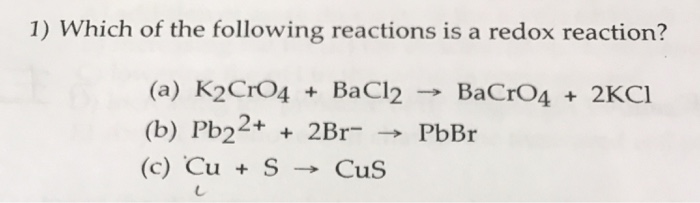 Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 BaCl 2 (aq) + 1 K 2 CrO 4 (aq) = 1 KCl (aq) + 1 BaCrO 4 (s) For each element, we check if the number …
Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 BaCl 2 (aq) + 1 K 2 CrO 4 (aq) = 1 KCl (aq) + 1 BaCrO 4 (s) For each element, we check if the number …
 Solved and balanced chemical equation K2CrO4 + BaCl2 → 2 KCl + BaCrO4 with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation K2CrO4 + BaCl2 → 2 KCl + BaCrO4 with completed products. Application for completing products and balancing equations.
 Word Equation. K2CrO4 + BaCl2 = KCl + BaCrO4 is a Double Displacement (Metathesis) reaction where one mole of aqueous Potassium Chromate [K 2 CrO 4] and one mole of aqueous …
Word Equation. K2CrO4 + BaCl2 = KCl + BaCrO4 is a Double Displacement (Metathesis) reaction where one mole of aqueous Potassium Chromate [K 2 CrO 4] and one mole of aqueous …
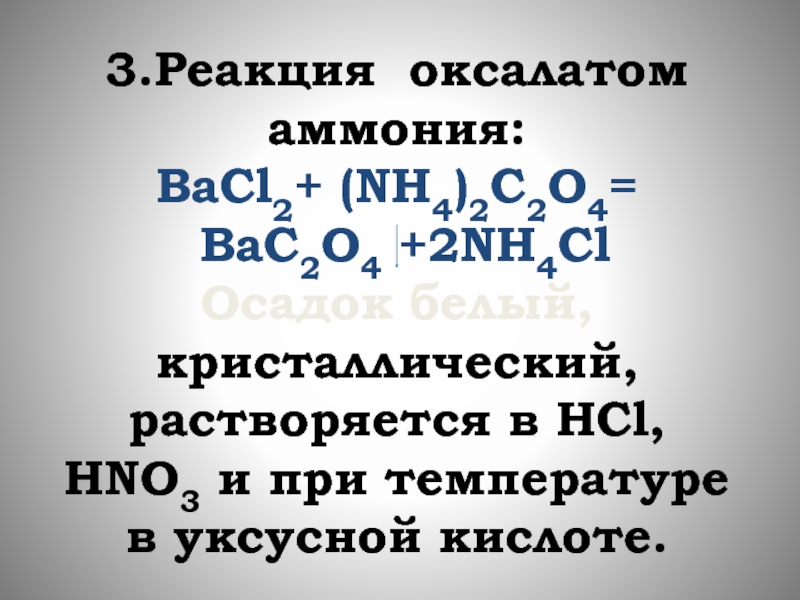
 Phản ứng BaCl 2 + K 2 CrO 4 tạo ra kết tủa BaCrO 4 thuộc loại phản ứng trao đổi đã được cân bằng chính xác và chi tiết nhất. Bên cạnh đó là một số bài tập có liên quan về BaCl 2 có lời …
Phản ứng BaCl 2 + K 2 CrO 4 tạo ra kết tủa BaCrO 4 thuộc loại phản ứng trao đổi đã được cân bằng chính xác và chi tiết nhất. Bên cạnh đó là một số bài tập có liên quan về BaCl 2 có lời …
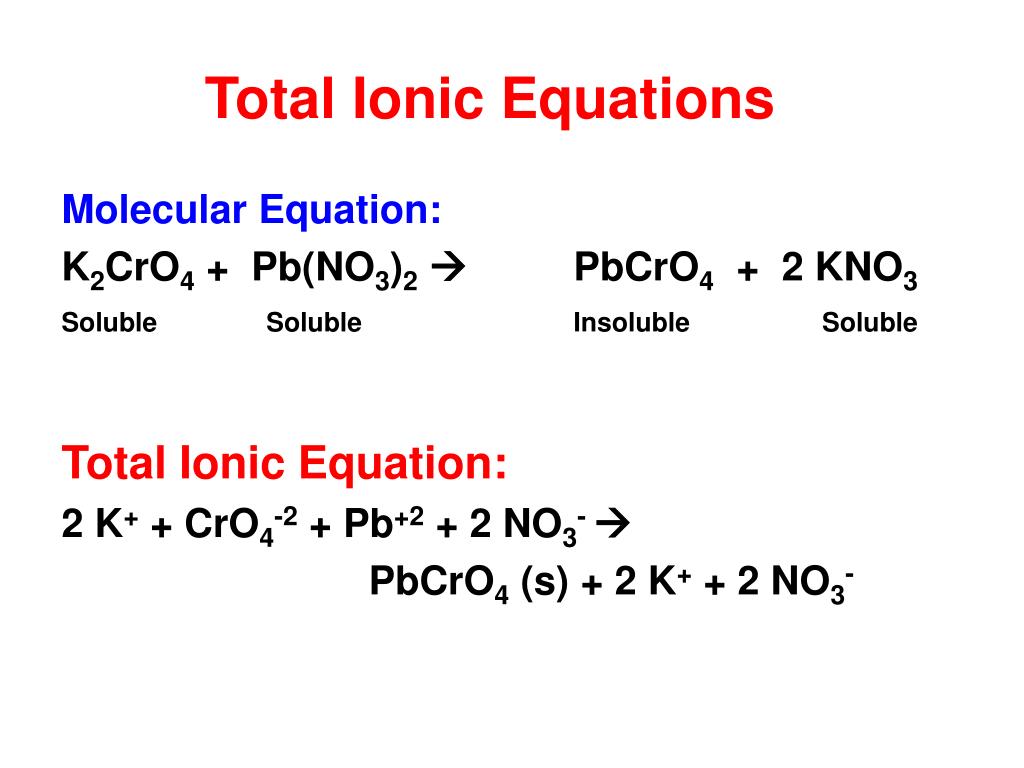
 There are three main steps for writing the net ionic equation for BaCl2 + K2CrO4 = BaCrO4 + KCl (Barium chloride + Potassium chromate). First, we balance the.
There are three main steps for writing the net ionic equation for BaCl2 + K2CrO4 = BaCrO4 + KCl (Barium chloride + Potassium chromate). First, we balance the.
 Construct the rate of reaction expression for: K_2CrO_4 + BaCl_2 KCl + BaCrO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for …
Construct the rate of reaction expression for: K_2CrO_4 + BaCl_2 KCl + BaCrO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for …
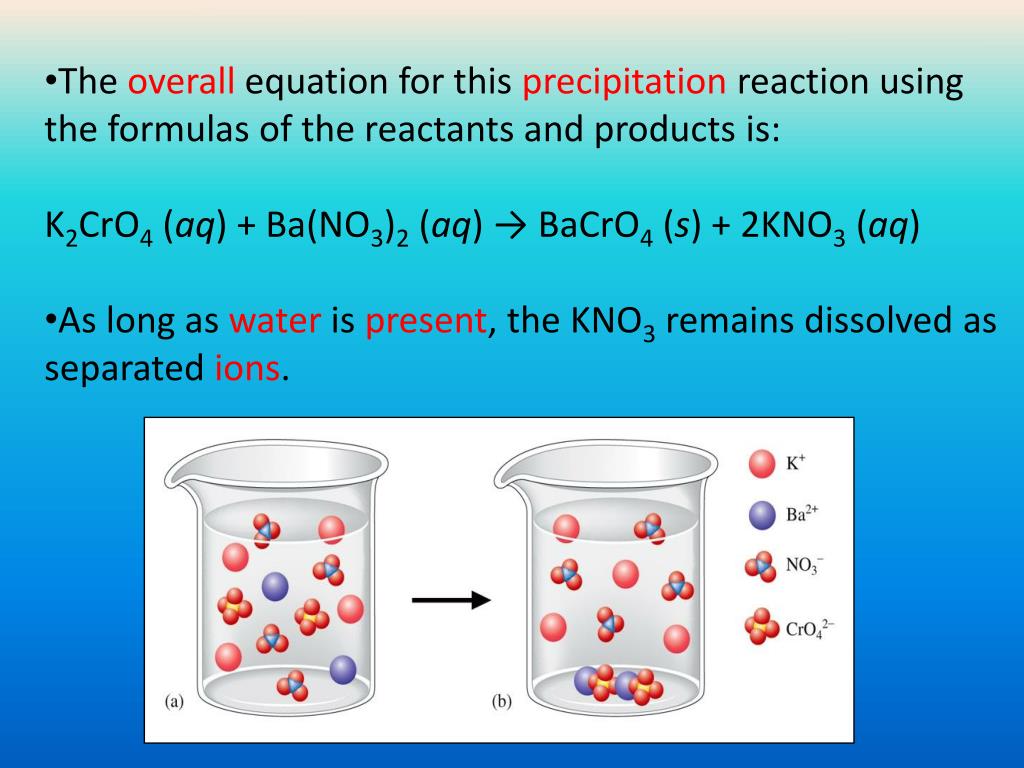
 When mixed, solutions of barium chloride, BaCl2, and potassium. chromate, K2CrO4, form a yellow precipitate of barium chromate, BaCrO4. The balanced equation is: BaCl2(aq) + K2CrO4(aq) →. BaCrO4(s) + 2KCl(aq) How many …
When mixed, solutions of barium chloride, BaCl2, and potassium. chromate, K2CrO4, form a yellow precipitate of barium chromate, BaCrO4. The balanced equation is: BaCl2(aq) + K2CrO4(aq) →. BaCrO4(s) + 2KCl(aq) How many …
 The reaction between barium chloride and potassium chromate is a double displacement reaction. The chemical equation for the reaction is as follows: BaCl2 + K2CrO4 → BaCrO4 + 2KCl In …
The reaction between barium chloride and potassium chromate is a double displacement reaction. The chemical equation for the reaction is as follows: BaCl2 + K2CrO4 → BaCrO4 + 2KCl In …
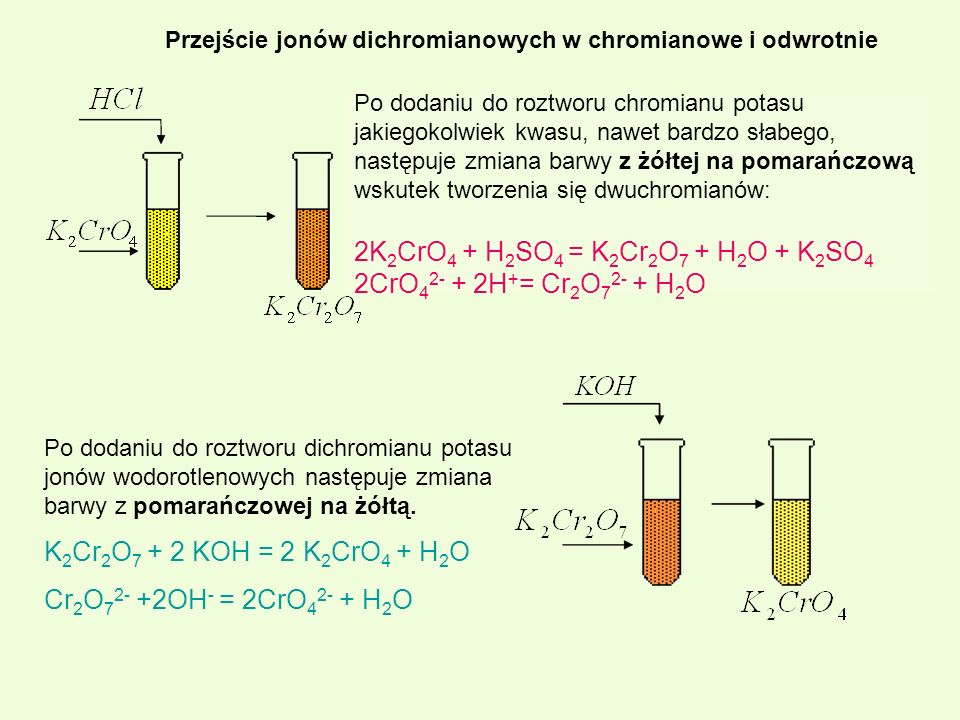
 When mixed, solutions of barium chloride, BaCl2, and potassium chromate, K2CrO4, form a yellow precipitate of barium chromate, BaCrO4. The balanced equation is: BaCl2 (aq) + …
When mixed, solutions of barium chloride, BaCl2, and potassium chromate, K2CrO4, form a yellow precipitate of barium chromate, BaCrO4. The balanced equation is: BaCl2 (aq) + …
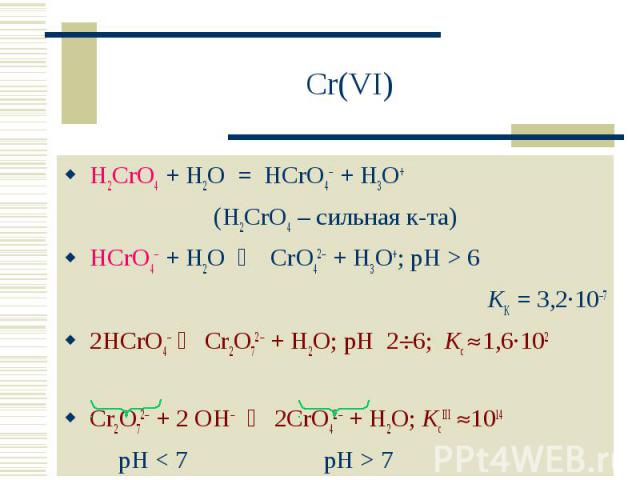
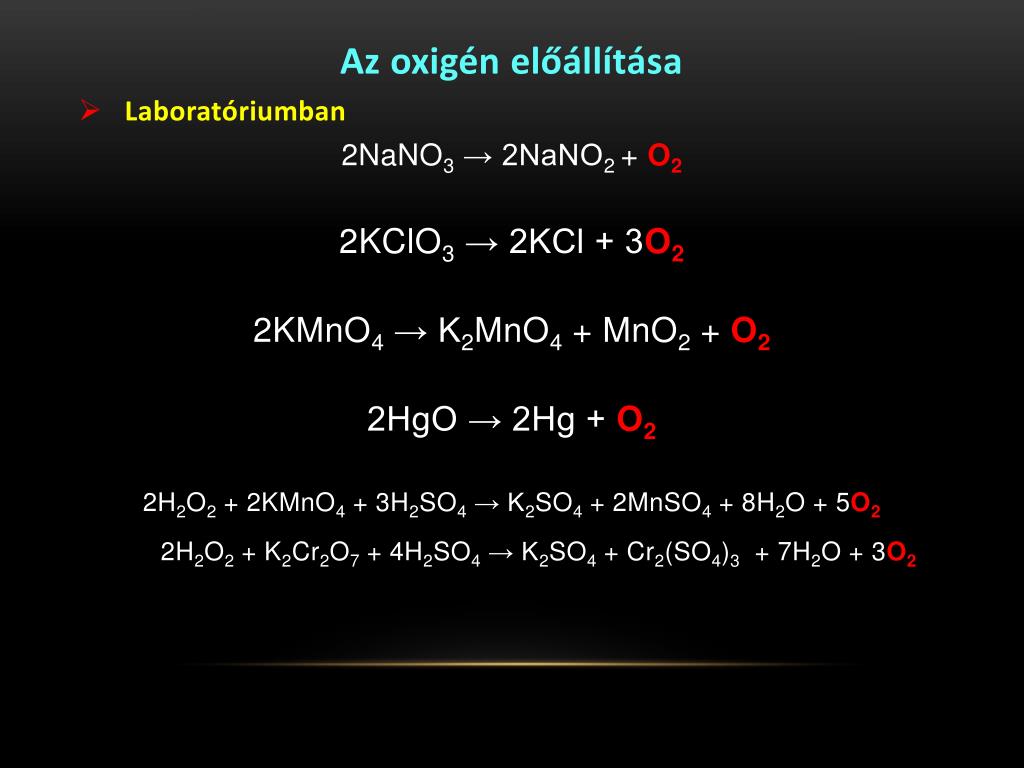
 Check the balance. Now, both sides have 4 H atoms and 2 O atoms. The equation is balanced. Balancing with algebraic method. This method uses algebraic equations to find the correct …
Check the balance. Now, both sides have 4 H atoms and 2 O atoms. The equation is balanced. Balancing with algebraic method. This method uses algebraic equations to find the correct …
 Phản ứng BaCl 2 + K 2 CrO 4 tạo ra kết tủa BaCrO 4 thuộc loại phản ứng trao đổi đã được cân bằng chính xác và chi tiết nhất. Bên cạnh đó là một số bài tập có liên quan về …
Phản ứng BaCl 2 + K 2 CrO 4 tạo ra kết tủa BaCrO 4 thuộc loại phản ứng trao đổi đã được cân bằng chính xác và chi tiết nhất. Bên cạnh đó là một số bài tập có liên quan về …
 Consider the reaction: BaCl2 + K2CrO4 → BaCrO4 + 2KCI a. How many grams of Barium chromate (253.33g/mol) can be obtained from 75.0mL of 0.150M BaCl2 solution? b. What …
Consider the reaction: BaCl2 + K2CrO4 → BaCrO4 + 2KCI a. How many grams of Barium chromate (253.33g/mol) can be obtained from 75.0mL of 0.150M BaCl2 solution? b. What …
 Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
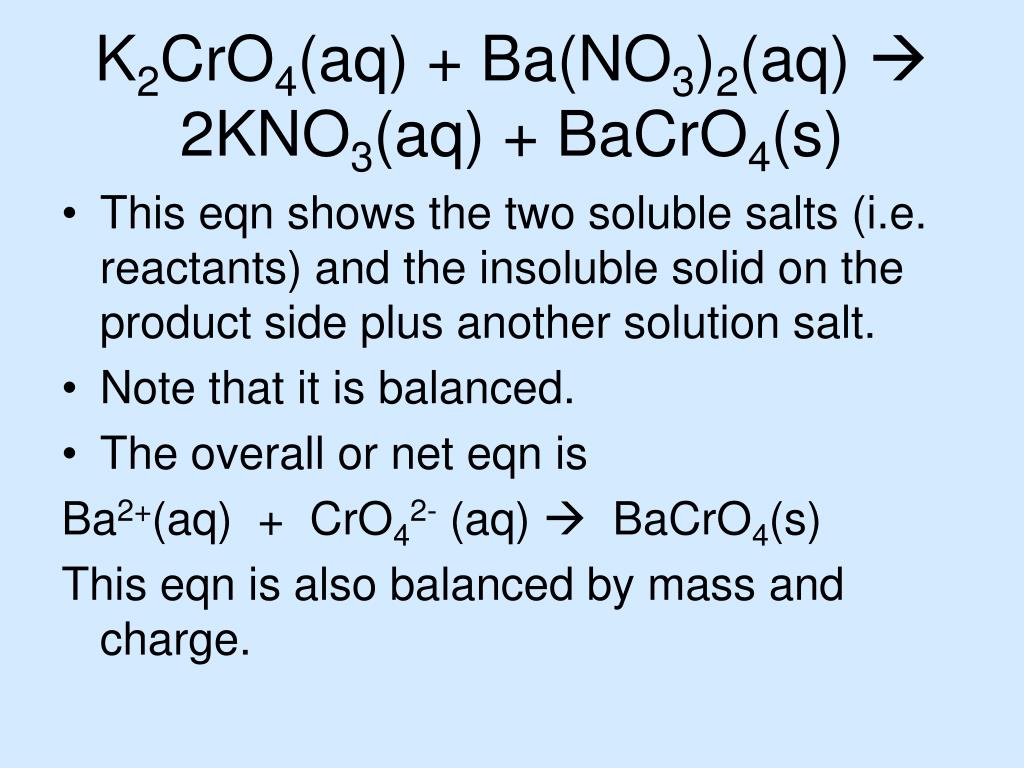
 Write the full balanced equation, the full ionic equation, and the net ionic equation for a solution of barium chloride, BaCl2, reacting with a solution of magnesium sulfate, MgSO4, to form the.
Write the full balanced equation, the full ionic equation, and the net ionic equation for a solution of barium chloride, BaCl2, reacting with a solution of magnesium sulfate, MgSO4, to form the.
Еще по теме:






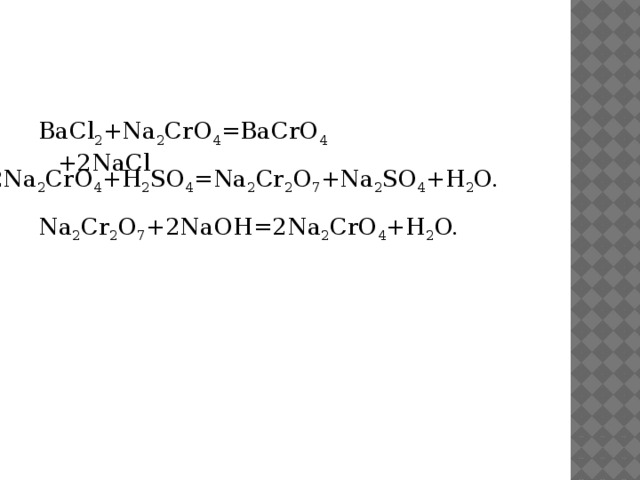





Еще по теме: