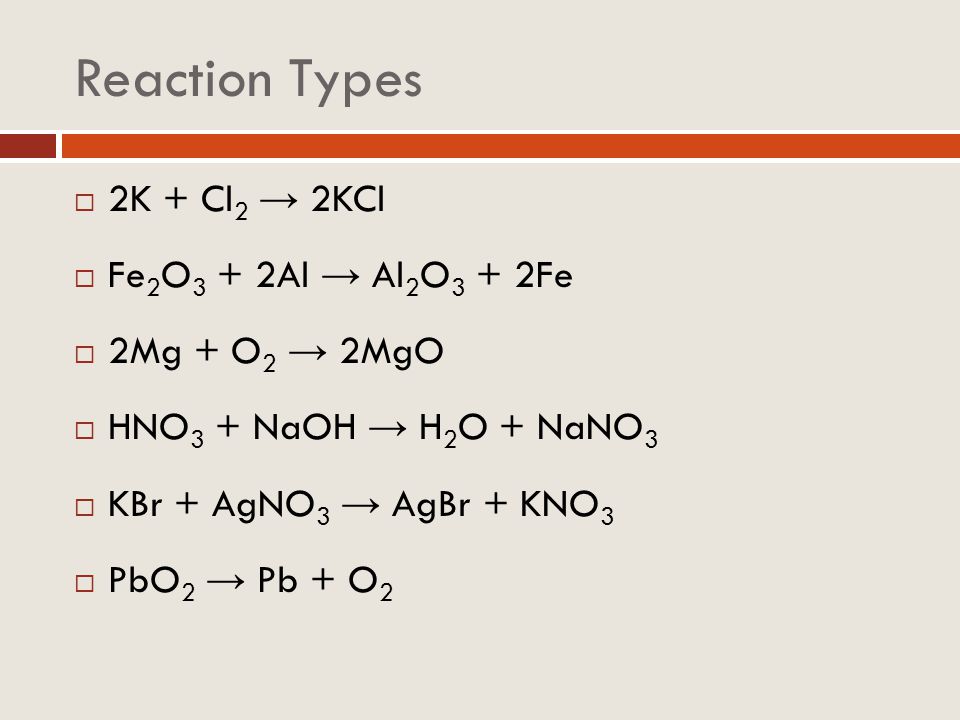
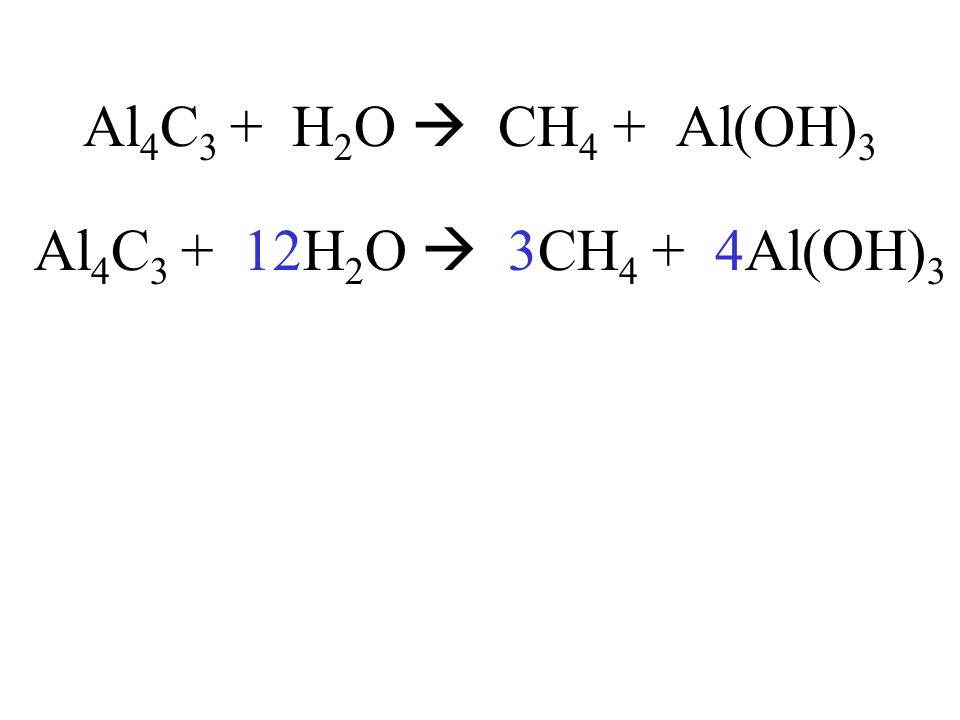Решенное и коэффициентами уравнение реакции 4 Al + 3 O2 → 2 Al2O3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 4 Al + 3 O2 → 2 Al2O3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Units: molar mass - g/mol, weight - g. Let's balance this equation using the inspection method. For each element, we check if the number of atoms is balanced on both sides of the equation. Al is …
Units: molar mass - g/mol, weight - g. Let's balance this equation using the inspection method. For each element, we check if the number of atoms is balanced on both sides of the equation. Al is …
 Since there is an equal number of each element in the reactants and products of 4Al + 3O2 = 2Al2O3, the equation is balanced.
Since there is an equal number of each element in the reactants and products of 4Al + 3O2 = 2Al2O3, the equation is balanced.

 Бесплатный калькулятор по Химии - Пошаговый расчет химических реакций и химических свойств
Бесплатный калькулятор по Химии - Пошаговый расчет химических реакций и химических свойств
 AI explanations are generated using OpenAI technology. AI generated content may present inaccurate or offensive content that does not represent Symbolab's view. Math can be an …
AI explanations are generated using OpenAI technology. AI generated content may present inaccurate or offensive content that does not represent Symbolab's view. Math can be an …
 This video is to explain how to prove that the formation of aluminum oxide (Al2O3) is a redox (oxidation-reduction) reaction.#chemistry #redox #oxidation #re.
This video is to explain how to prove that the formation of aluminum oxide (Al2O3) is a redox (oxidation-reduction) reaction.#chemistry #redox #oxidation #re.
 Study this chemical reaction: 4Al + 3O2 --> 2Al2O3 Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction. Your solution’s ready to go! …
Study this chemical reaction: 4Al + 3O2 --> 2Al2O3 Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction. Your solution’s ready to go! …
 Atomic level interpretation: 4 Al atoms combine with 3 O2 molecules to form 2 molecules of Al2O3. Mole level interpretation: Take what I said above and use "moles" instead …
Atomic level interpretation: 4 Al atoms combine with 3 O2 molecules to form 2 molecules of Al2O3. Mole level interpretation: Take what I said above and use "moles" instead …
 The balanced equation tells us we get 2 moles of product for every 3 moles of O 2. If we assume that there is more than enough aluminum as a reactant, the moles of product …
The balanced equation tells us we get 2 moles of product for every 3 moles of O 2. If we assume that there is more than enough aluminum as a reactant, the moles of product …
 Consider the balanced equation: 4Al + 3O2 → 2Al2O3 What is the sum of the stoichiometric coefficients? a. 4 b. 8 c. 9 d. 10 e. 20. Your solution’s ready to go! Our expert help has broken …
Consider the balanced equation: 4Al + 3O2 → 2Al2O3 What is the sum of the stoichiometric coefficients? a. 4 b. 8 c. 9 d. 10 e. 20. Your solution’s ready to go! Our expert help has broken …
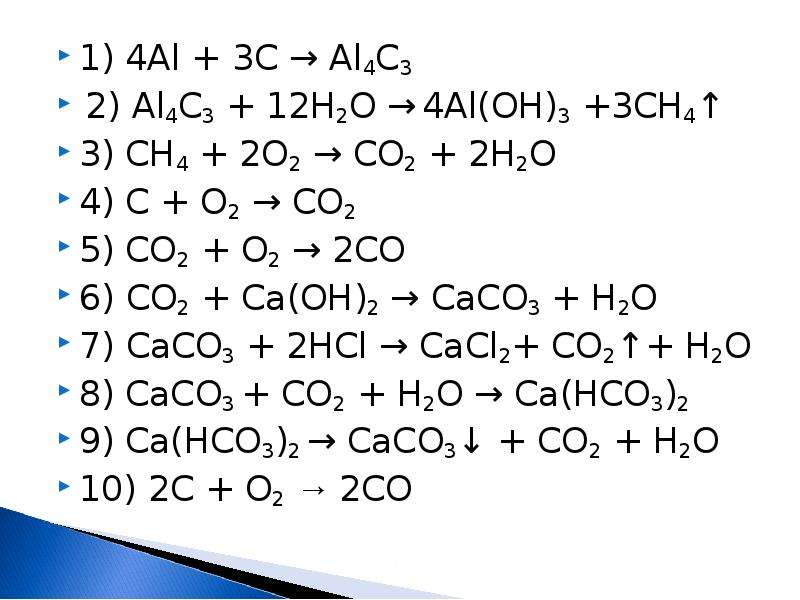
 Al + 3O2 = 2Al2O3 уравниваем алюминий, ставя перед Al 4 получается 4A l+ 3O2 = 2Al2O3
Al + 3O2 = 2Al2O3 уравниваем алюминий, ставя перед Al 4 получается 4A l+ 3O2 = 2Al2O3
 To be balanced, every element in (2Al2O3) = (4Al) + (3O2) must have the same number of atoms on each side of the equation. When using the inspection method (also known as the trial-and …
To be balanced, every element in (2Al2O3) = (4Al) + (3O2) must have the same number of atoms on each side of the equation. When using the inspection method (also known as the trial-and …
 For the balanced chemical reaction 4Al + 3O2 → 2Al2O3 complete the statement to properly convert from moles of oxygen to moles of aluminum oxide. Use numbers as answers. The …
For the balanced chemical reaction 4Al + 3O2 → 2Al2O3 complete the statement to properly convert from moles of oxygen to moles of aluminum oxide. Use numbers as answers. The …
 The given reaction is 4Al+ 3O2----->2Al2O3 theoretical moles ratio of Al to O2= 4/3= 1.33 if the actual moles ratio is more than 1 …View the full answer
The given reaction is 4Al+ 3O2----->2Al2O3 theoretical moles ratio of Al to O2= 4/3= 1.33 if the actual moles ratio is more than 1 …View the full answer
 1 (4Al) + 1 (3O 2) = 1 (2Al 2 O 3) For each element, we check if the number of atoms is balanced on both sides of the equation. Al is balanced: 4 atoms in reagents and 4 atoms in products.
1 (4Al) + 1 (3O 2) = 1 (2Al 2 O 3) For each element, we check if the number of atoms is balanced on both sides of the equation. Al is balanced: 4 atoms in reagents and 4 atoms in products.
Еще по теме:




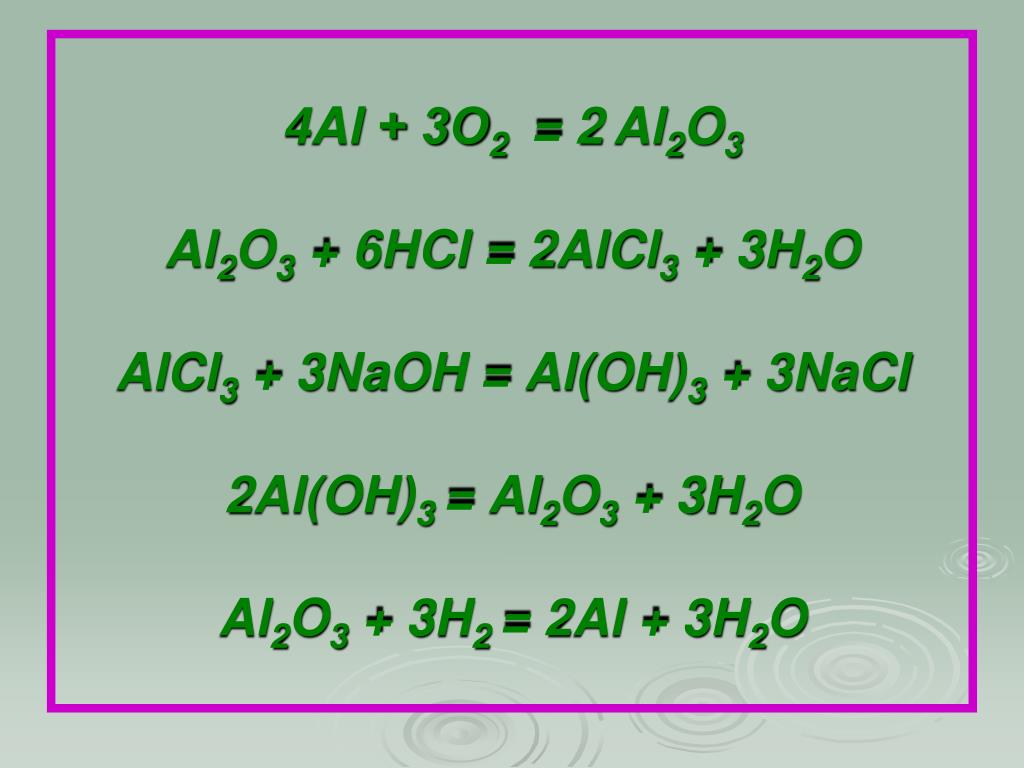





Еще по теме: