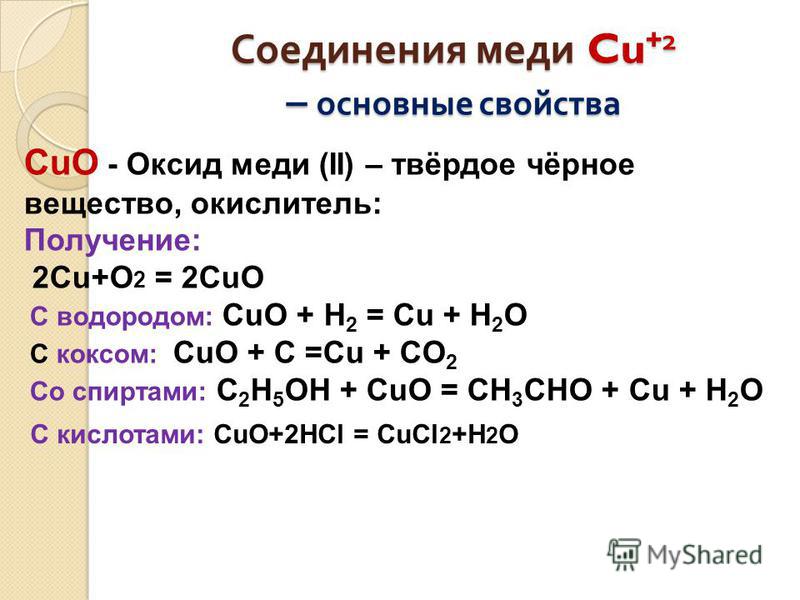
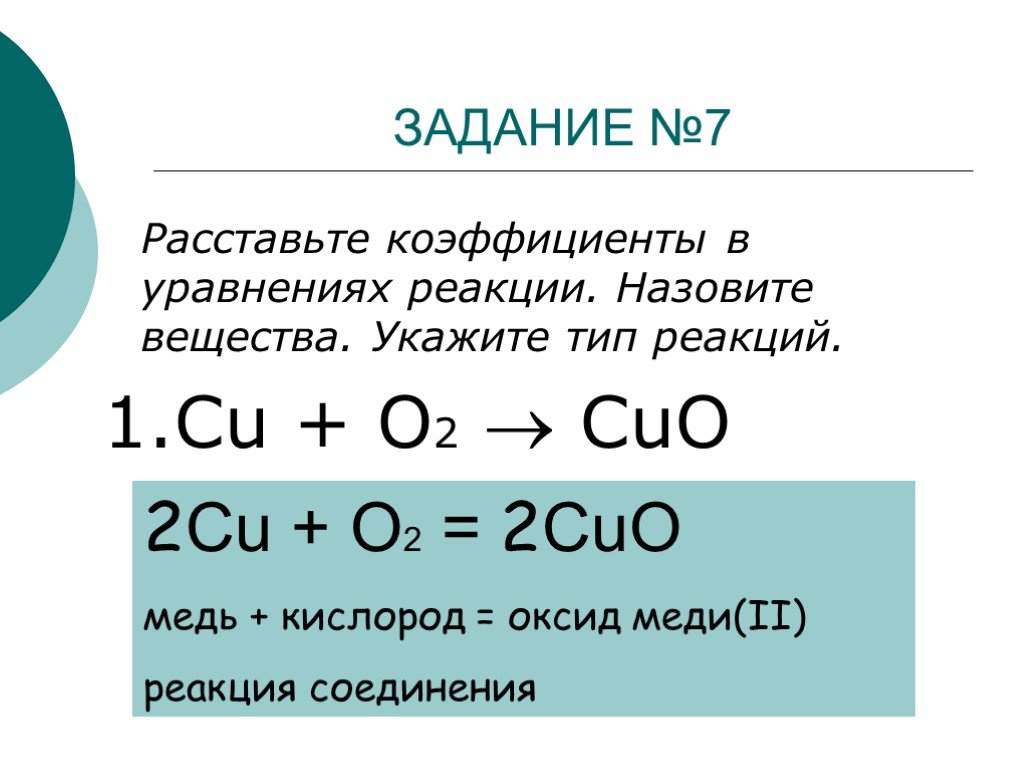
 Решенное и коэффициентами уравнение реакции 2 Cu + O2 → 2 CuO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 Cu + O2 → 2 CuO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Cu + O2 = CuO is a Synthesis reaction where two moles of Copper [Cu] and one mole of Dioxygen [O 2] combine to form two moles of Copper(Ii) Oxide [CuO]
Cu + O2 = CuO is a Synthesis reaction where two moles of Copper [Cu] and one mole of Dioxygen [O 2] combine to form two moles of Copper(Ii) Oxide [CuO]
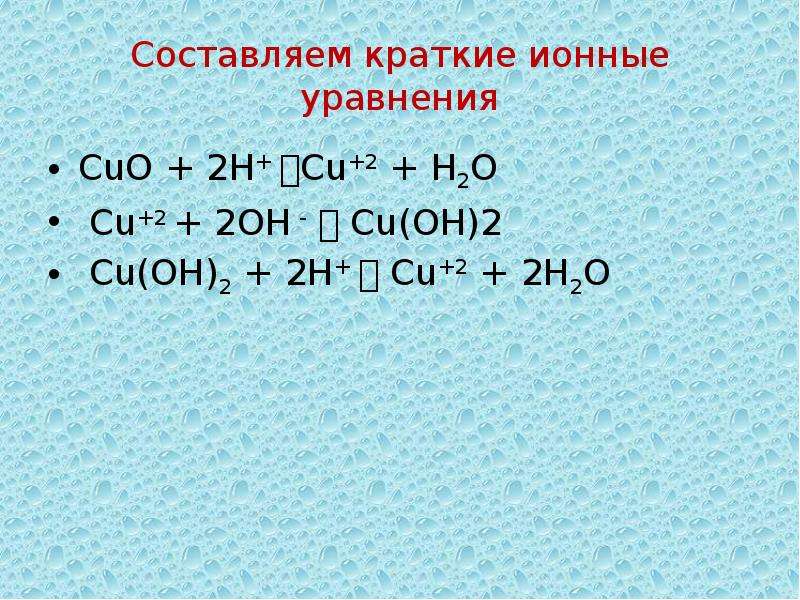
 1 Cu + 1 O 2 = 1 CuO For each element, we check if the number of atoms is balanced on both sides of the equation. Cu is balanced: 1 atom in reagents and 1 atom in products.
1 Cu + 1 O 2 = 1 CuO For each element, we check if the number of atoms is balanced on both sides of the equation. Cu is balanced: 1 atom in reagents and 1 atom in products.
 Cu2 + O2 = CuO is a Synthesis reaction where one mole of Dinuclear Copper Ion [Cu 2] and one mole of Dioxygen [O 2] combine to form two moles of Copper(Ii) Oxide [CuO]
Cu2 + O2 = CuO is a Synthesis reaction where one mole of Dinuclear Copper Ion [Cu 2] and one mole of Dioxygen [O 2] combine to form two moles of Copper(Ii) Oxide [CuO]
 As the oxygen atoms are maximum in number i.e 2 on the reactant side. To balance the oxygen atom, oxygen needs to be multiplied by “2” on the product side; On balancing oxygen on both …
As the oxygen atoms are maximum in number i.e 2 on the reactant side. To balance the oxygen atom, oxygen needs to be multiplied by “2” on the product side; On balancing oxygen on both …
 To balance Cu + O2 = CuO you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change …
To balance Cu + O2 = CuO you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change …
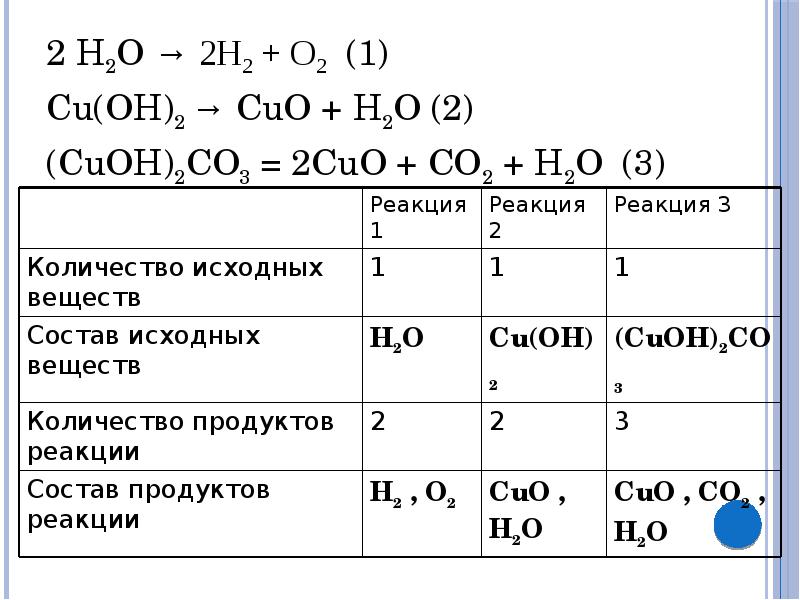
 Решенное и коэффициентами уравнение реакции 2 Cu2O + O2 → 4 CuO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 Cu2O + O2 → 4 CuO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 CuO = Cu + O2 is a Decomposition reaction where two moles of Copper(Ii) Oxide [CuO] decomposes into two moles of Copper [Cu] and one mole of Dioxygen [O 2]
CuO = Cu + O2 is a Decomposition reaction where two moles of Copper(Ii) Oxide [CuO] decomposes into two moles of Copper [Cu] and one mole of Dioxygen [O 2]
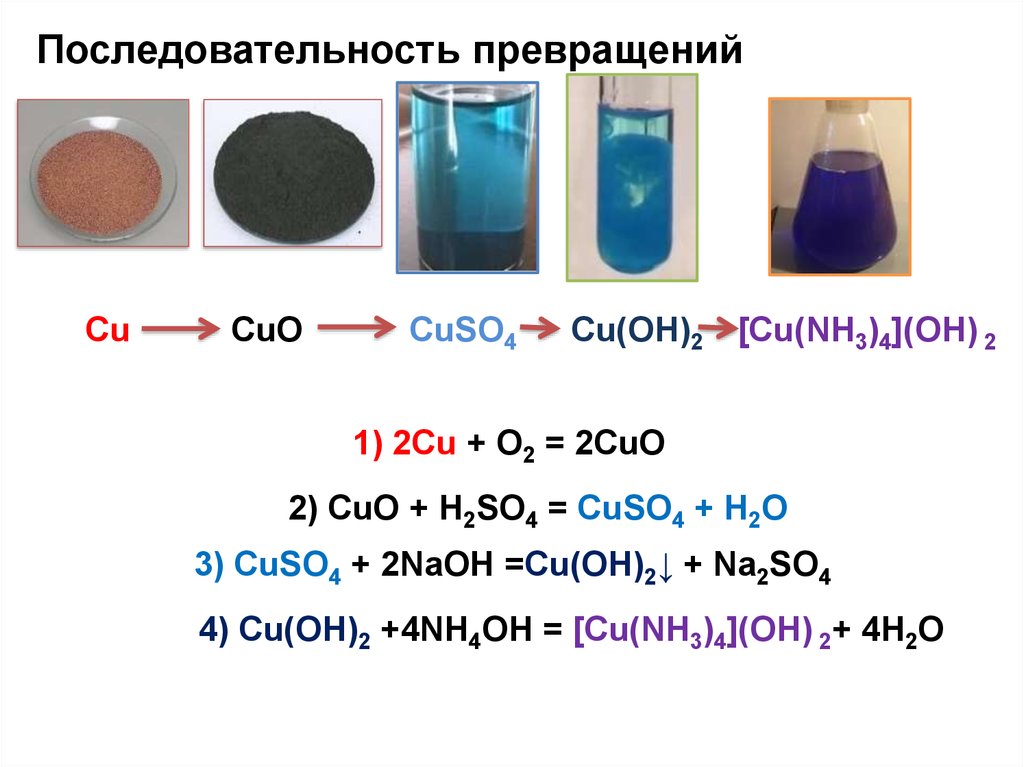
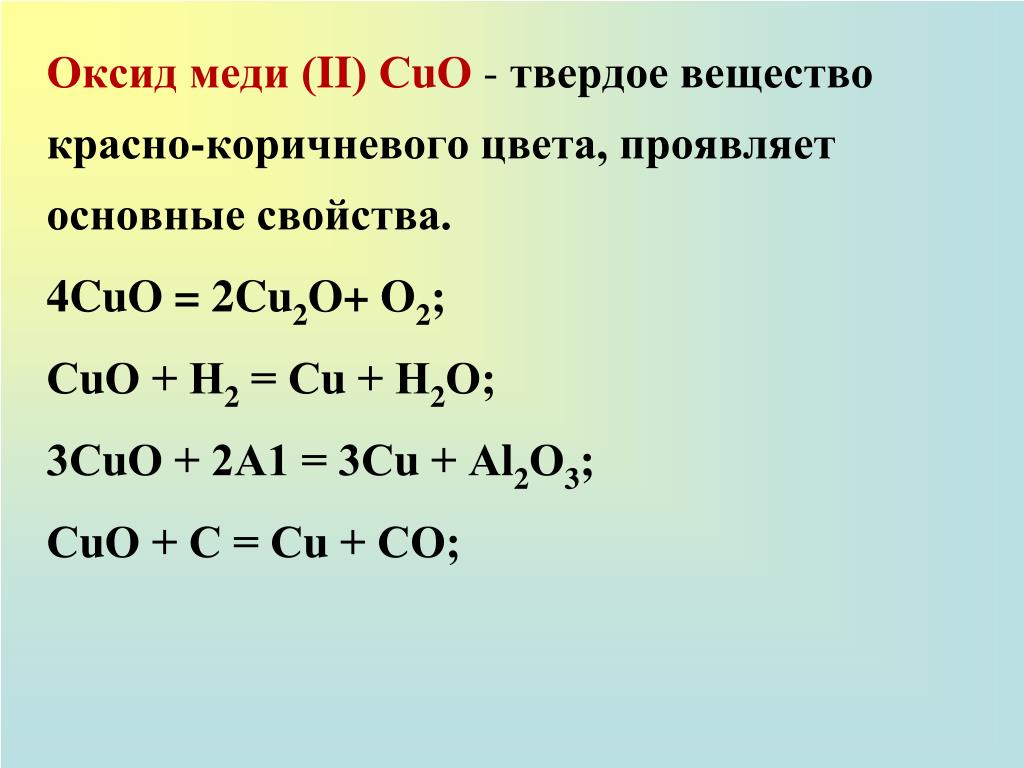
 Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu 2 O or copper(I) oxide (cuprous …
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu 2 O or copper(I) oxide (cuprous …
 A comparison of the Cu 2p NEXAFS spectra of metallic copper, CuO, and Cu 2 O with a low admixture of CuO (Figure 9 a) shows that the Cu 2p spectra make it possible to …
A comparison of the Cu 2p NEXAFS spectra of metallic copper, CuO, and Cu 2 O with a low admixture of CuO (Figure 9 a) shows that the Cu 2p spectra make it possible to …
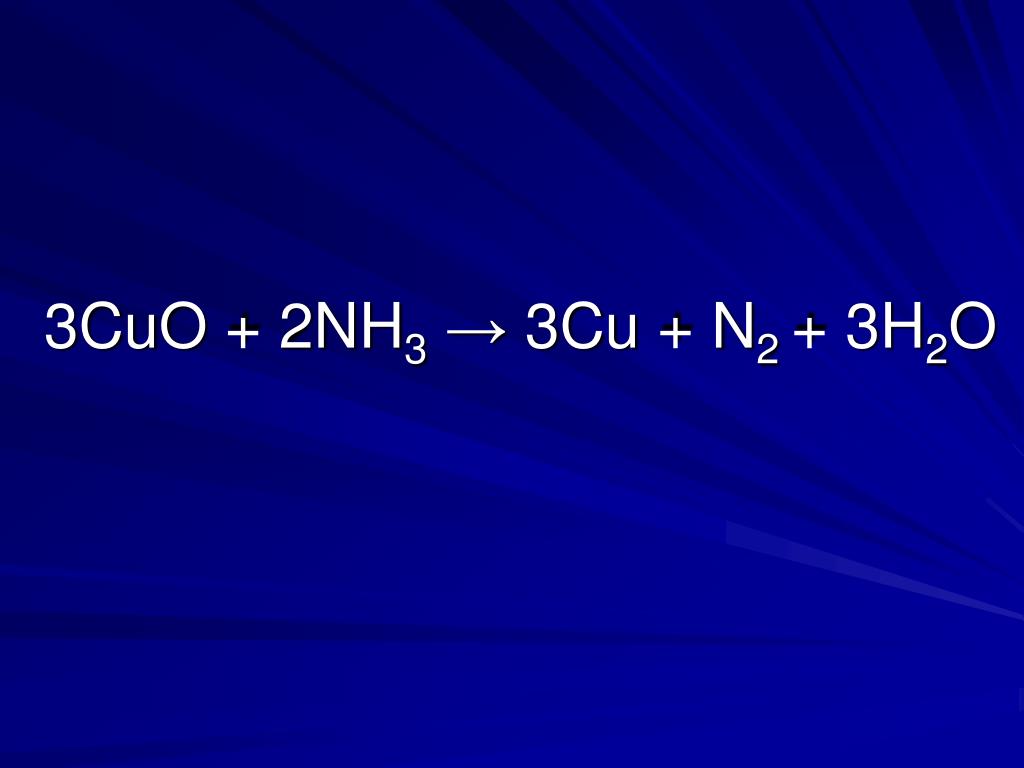
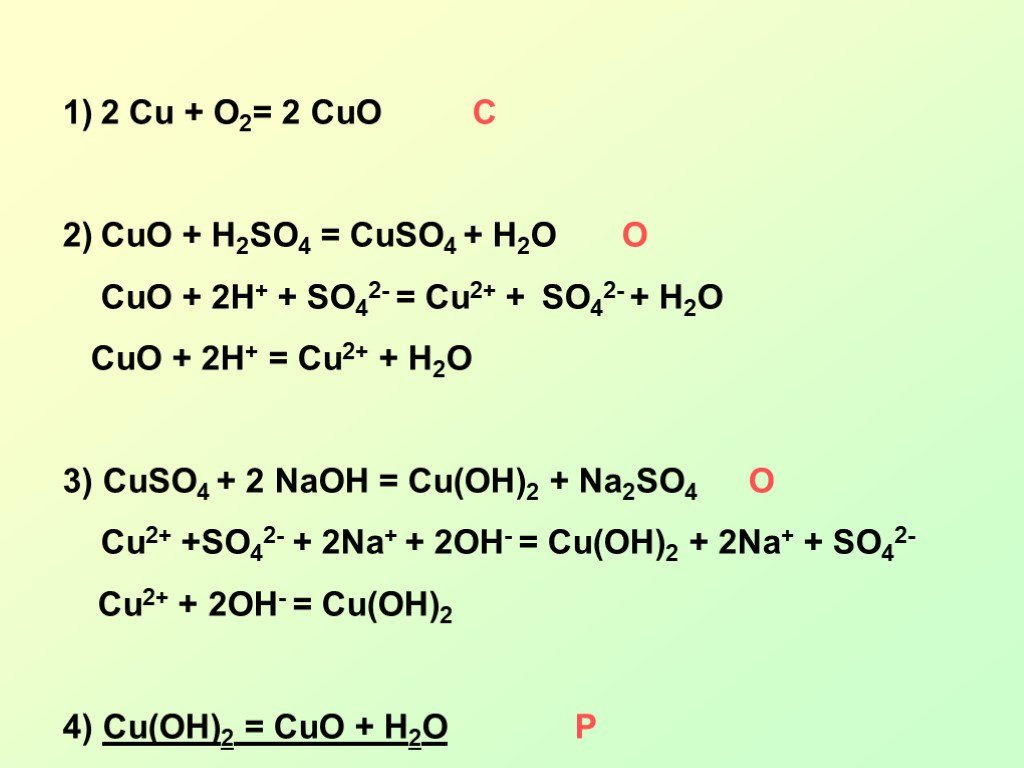
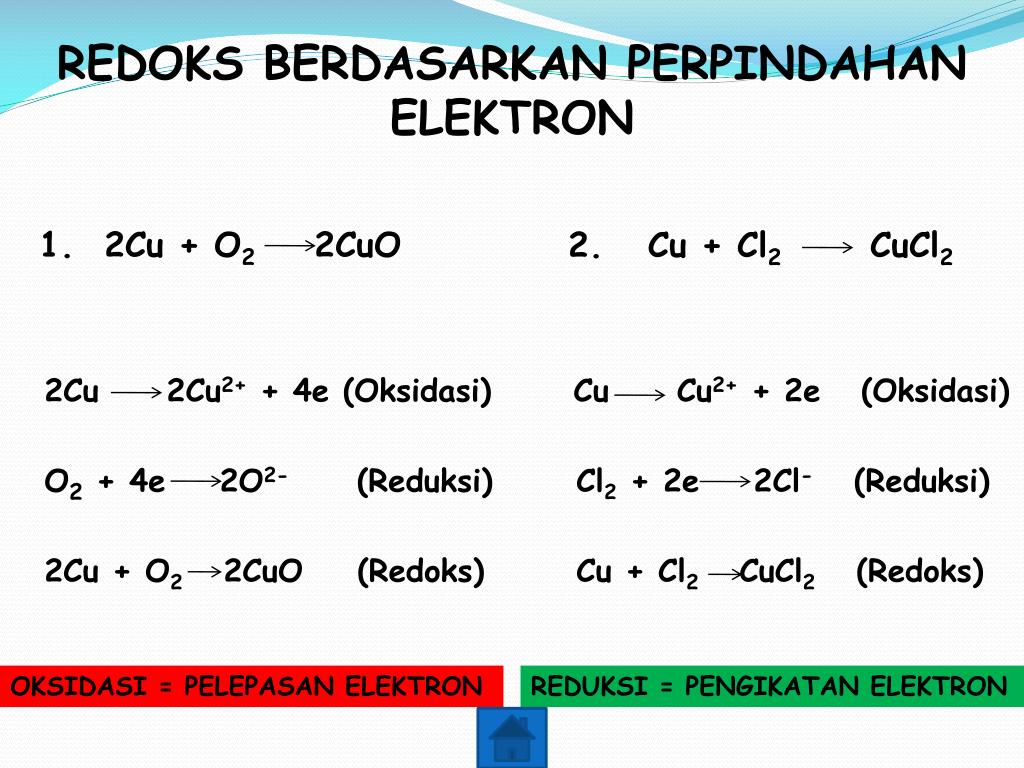
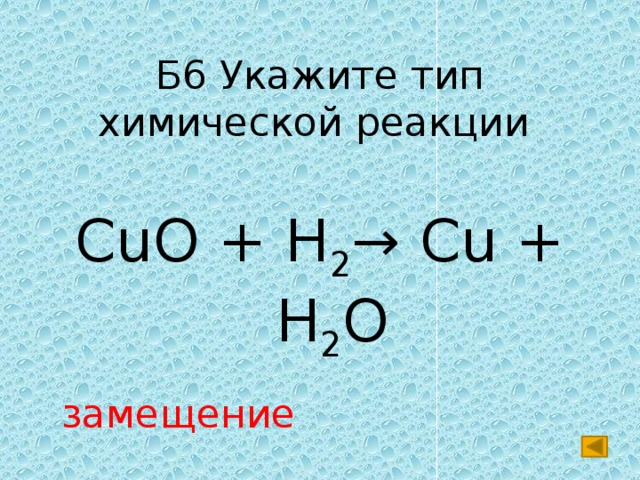
 2Cu + O2 = 2CuO is a redox reaction where Cu is oxidized and O is reduced. Cu is a reducing agent (i.e. it lost electrons) and O 2 is a oxidizing agent (i.e. it gained electrons).
2Cu + O2 = 2CuO is a redox reaction where Cu is oxidized and O is reduced. Cu is a reducing agent (i.e. it lost electrons) and O 2 is a oxidizing agent (i.e. it gained electrons).
 Results proved a strong synergistic effect – the coexistence of redox pairs Ce 4+ /Ce 3+-Cu 2+ /Cu +, the formation of oxygen vacancies, as well as the presence of superficial …
Results proved a strong synergistic effect – the coexistence of redox pairs Ce 4+ /Ce 3+-Cu 2+ /Cu +, the formation of oxygen vacancies, as well as the presence of superficial …
 Perovskite oxides with Cu and Sn at the B-site are mainly used for CO 2 RR (150, 151). Because of the challenge of synthesizing pure phases of Cu-based perovskite oxides, …
Perovskite oxides with Cu and Sn at the B-site are mainly used for CO 2 RR (150, 151). Because of the challenge of synthesizing pure phases of Cu-based perovskite oxides, …
 In this study, CuCr 2 O 4 -based catalytic oxygen carriers are tailored for lattice oxygen participating in low-temperature methanol reforming. We found that the low …
In this study, CuCr 2 O 4 -based catalytic oxygen carriers are tailored for lattice oxygen participating in low-temperature methanol reforming. We found that the low …
 High porosity and large photoactive surface of photoelectrodes grown on nano-grained and conductive ceramics provide freestanding structures for applications in …
High porosity and large photoactive surface of photoelectrodes grown on nano-grained and conductive ceramics provide freestanding structures for applications in …
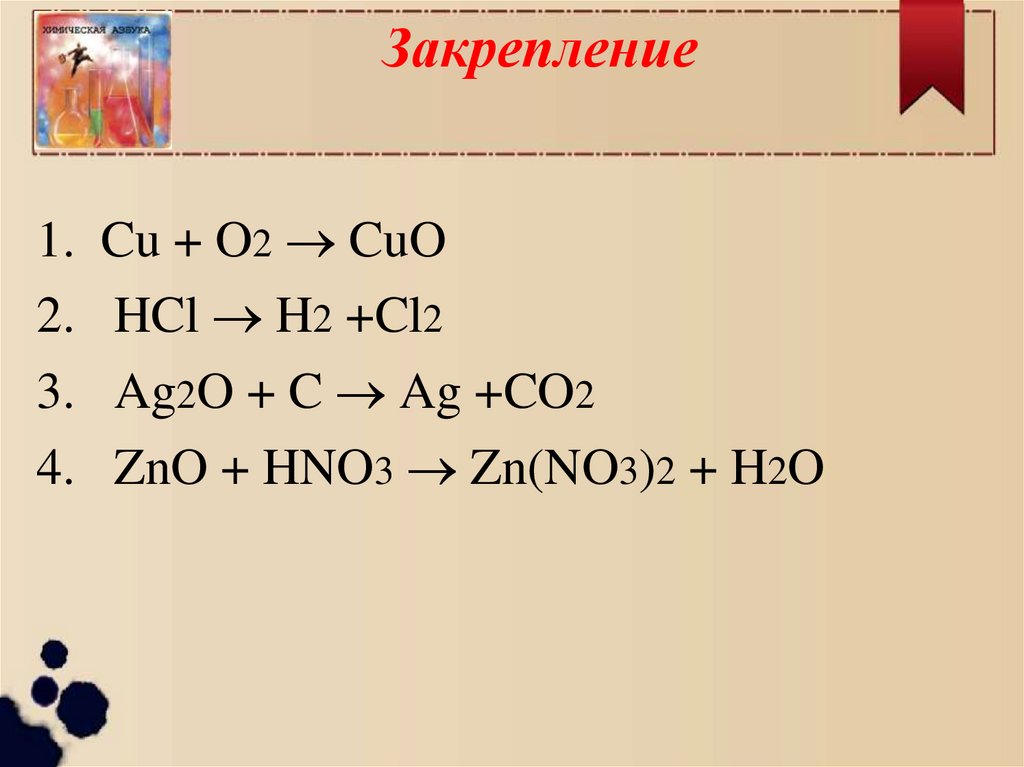
 1 cuo = 1 cu + 1 o 2 For each element, we check if the number of atoms is balanced on both sides of the equation. C is balanced: 1 atom in reagents and 1 atom in products.
1 cuo = 1 cu + 1 o 2 For each element, we check if the number of atoms is balanced on both sides of the equation. C is balanced: 1 atom in reagents and 1 atom in products.
Еще по теме:







Еще по теме: