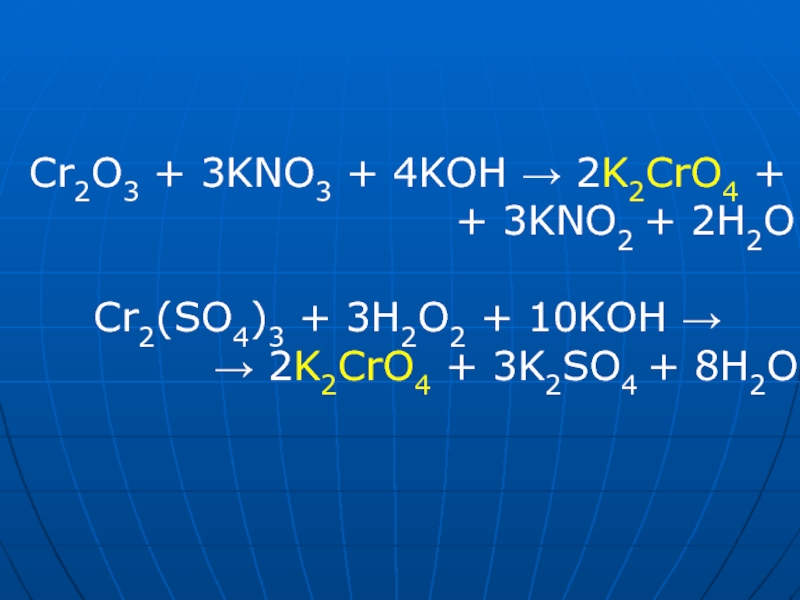
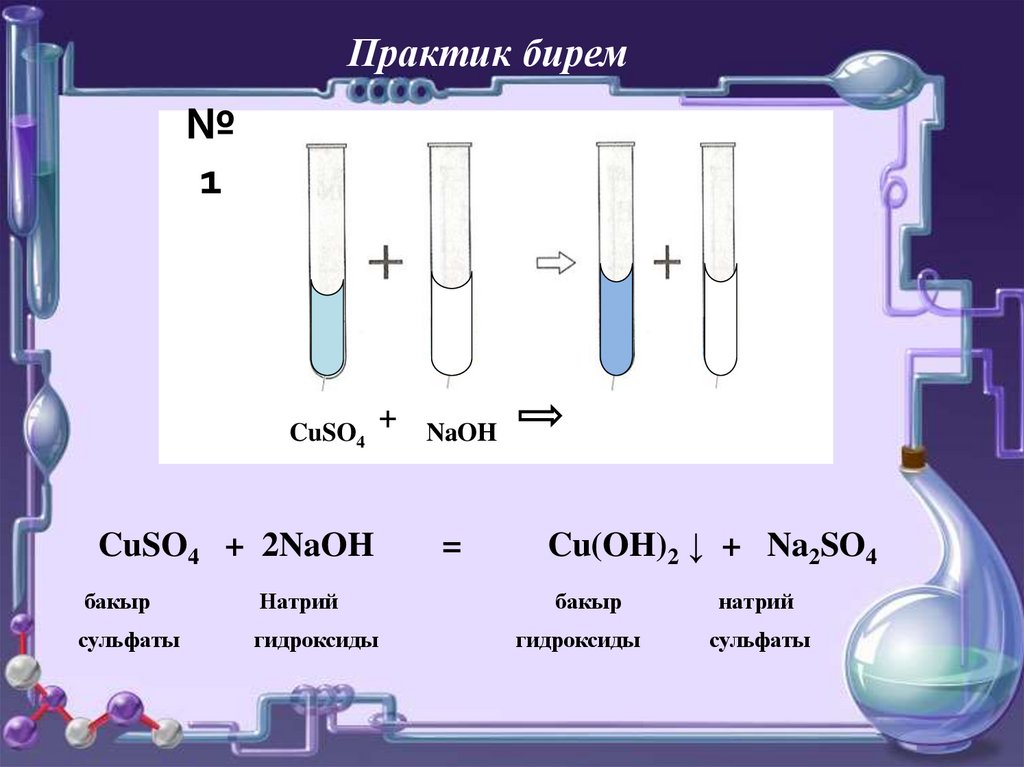
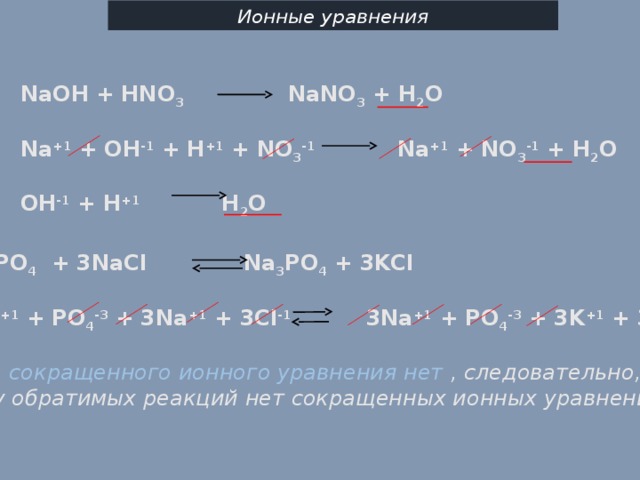
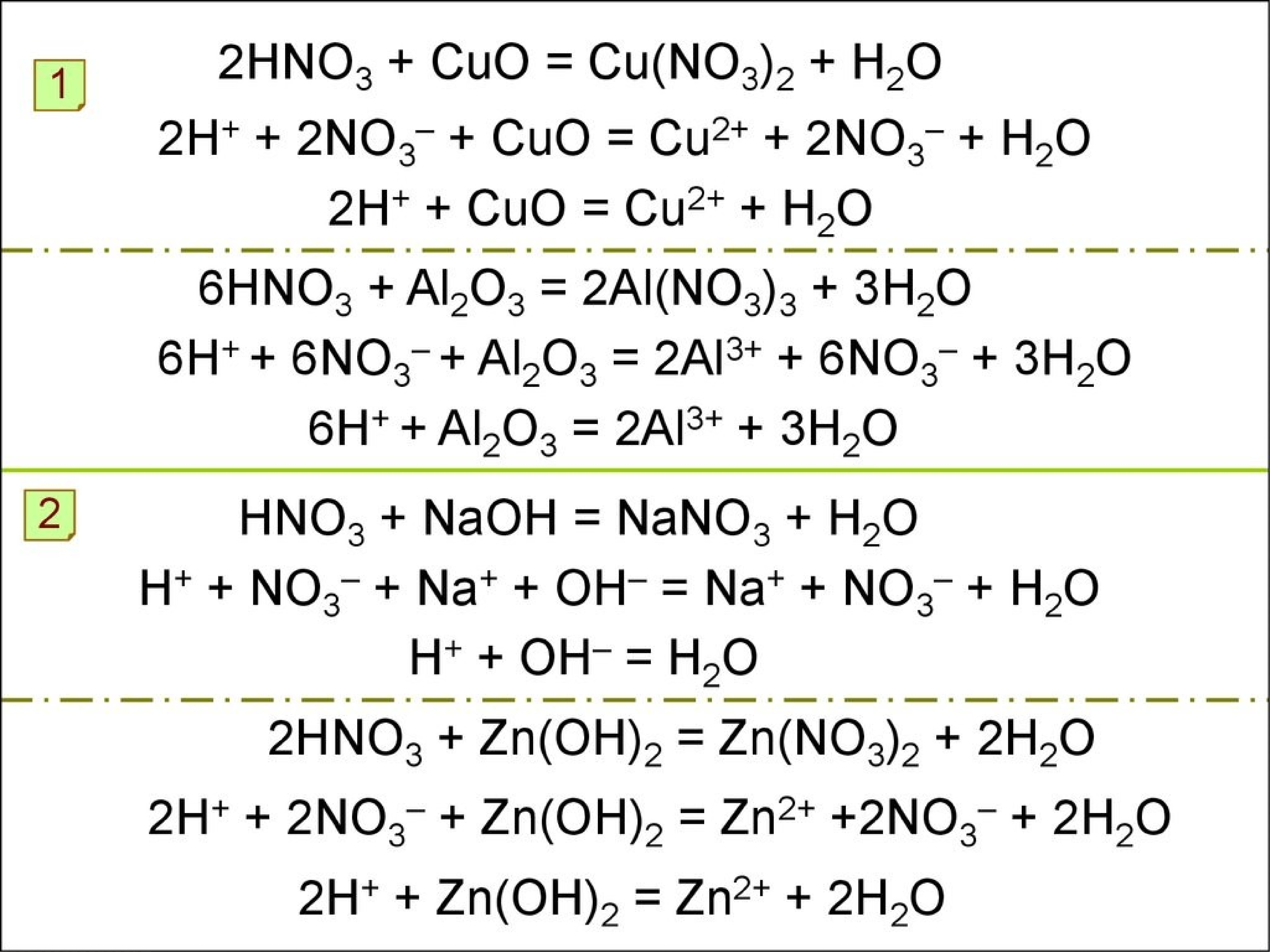
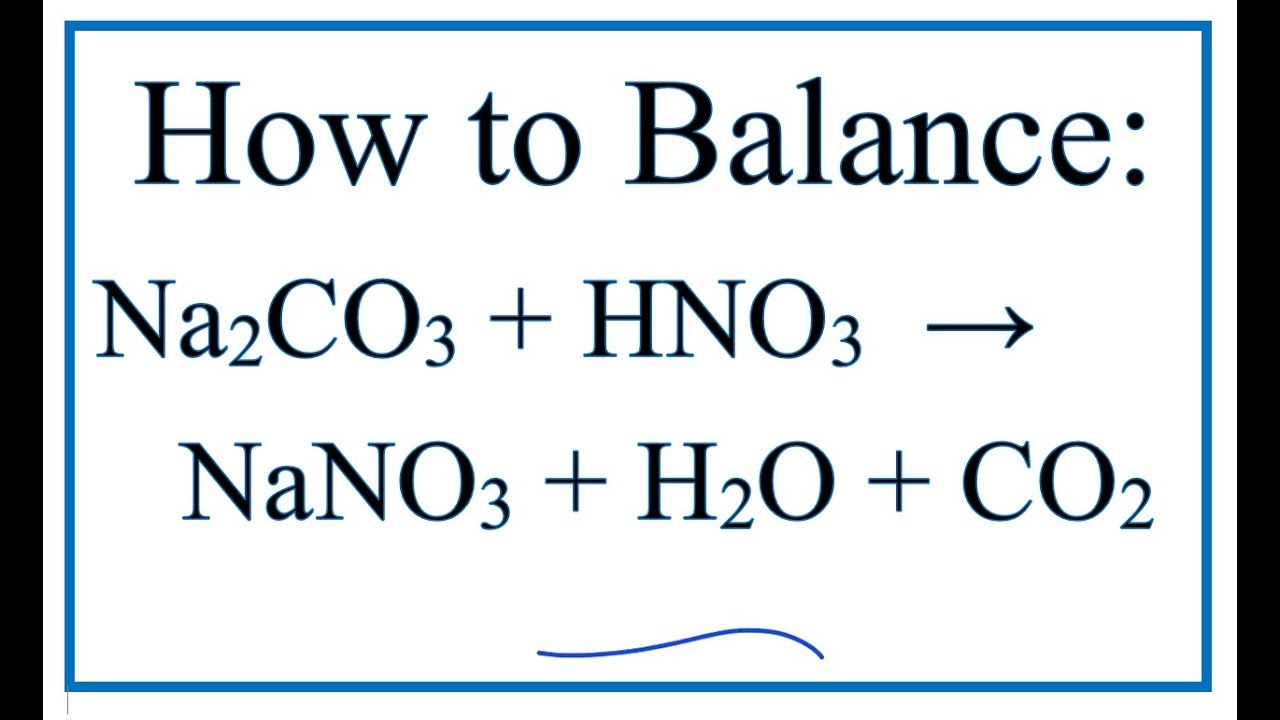
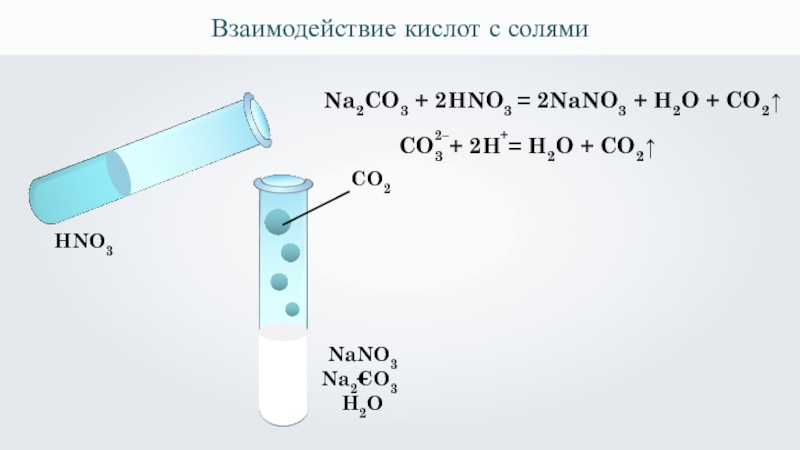
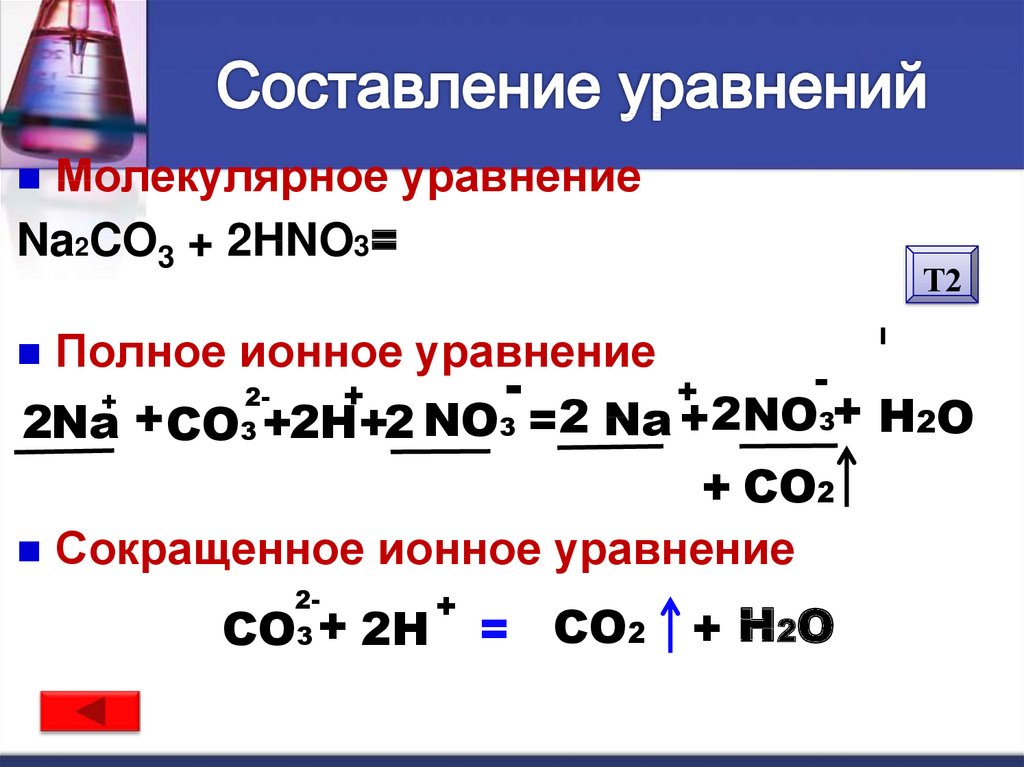
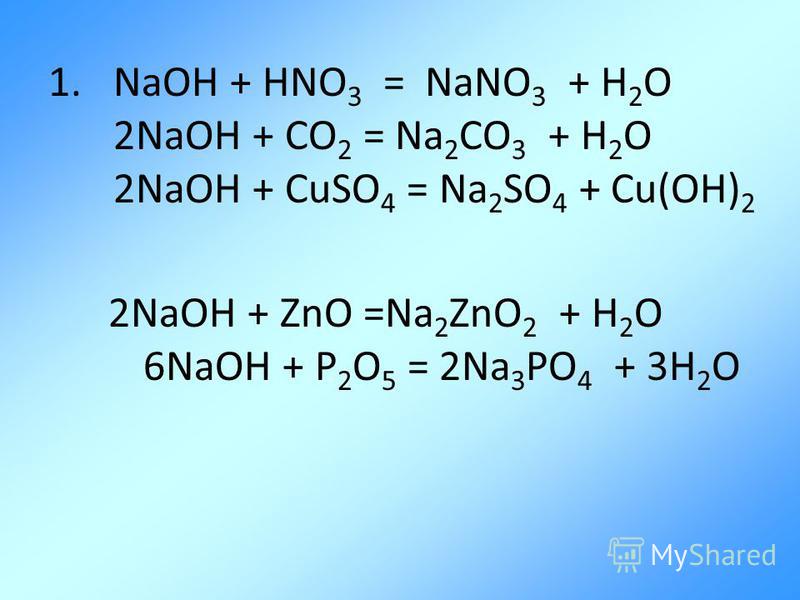
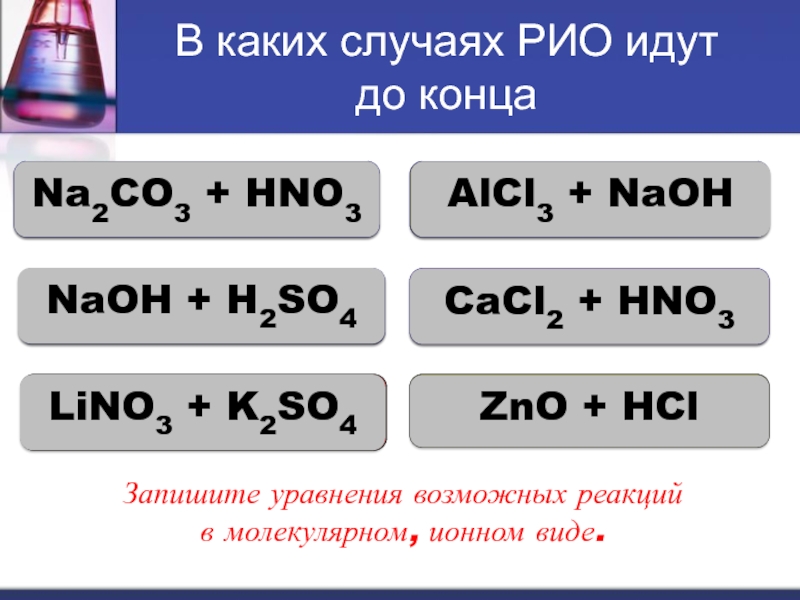
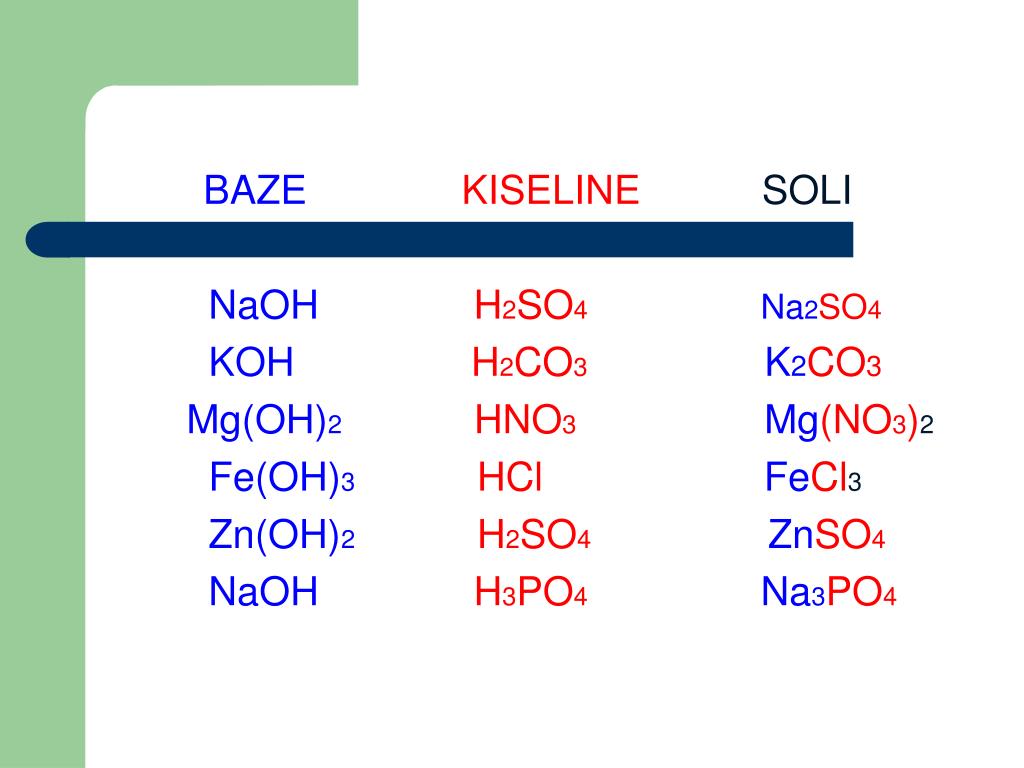
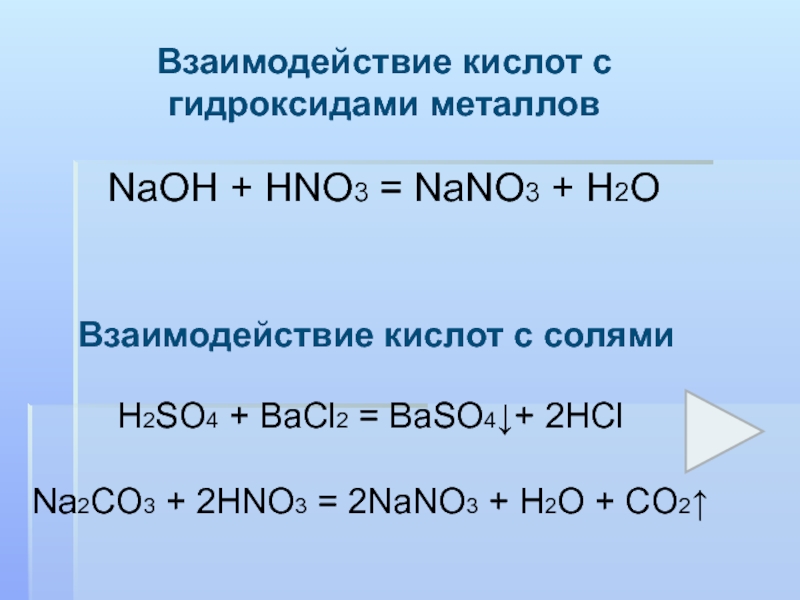
 The chemical reaction between nitric acid (HNO3) and sodium carbonate (Na2CO3) is: 2 HNO3 + Na2CO3 → 2 NaNO3 + H2O + CO2. In this reaction, nitric acid reacts with sodium carbonate to produce.
The chemical reaction between nitric acid (HNO3) and sodium carbonate (Na2CO3) is: 2 HNO3 + Na2CO3 → 2 NaNO3 + H2O + CO2. In this reaction, nitric acid reacts with sodium carbonate to produce.
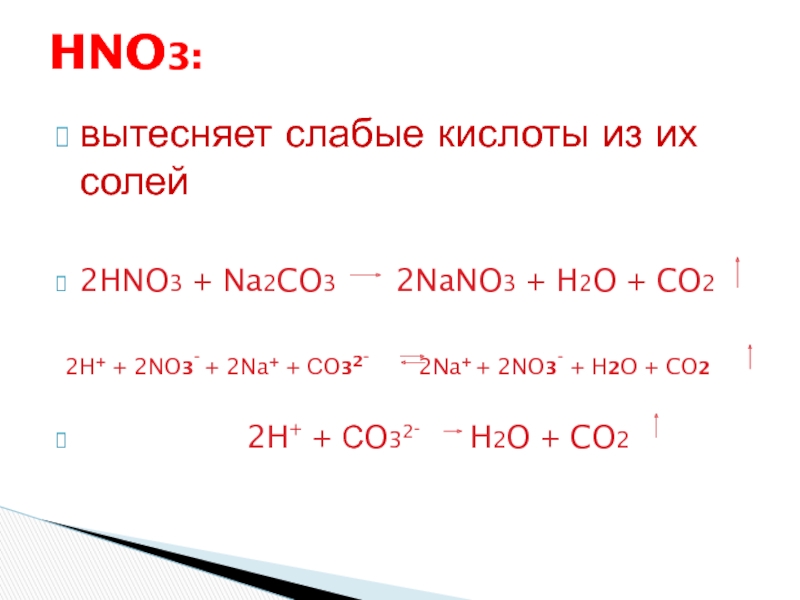 The chemical reaction between nitric acid (HNO3) and sodium carbonate (Na2CO3) is: 2 HNO3 + Na2CO3 → 2 NaNO3 + H2O + CO2. In this reaction, nitric acid reacts with sodium carbonate to produce.
The chemical reaction between nitric acid (HNO3) and sodium carbonate (Na2CO3) is: 2 HNO3 + Na2CO3 → 2 NaNO3 + H2O + CO2. In this reaction, nitric acid reacts with sodium carbonate to produce.
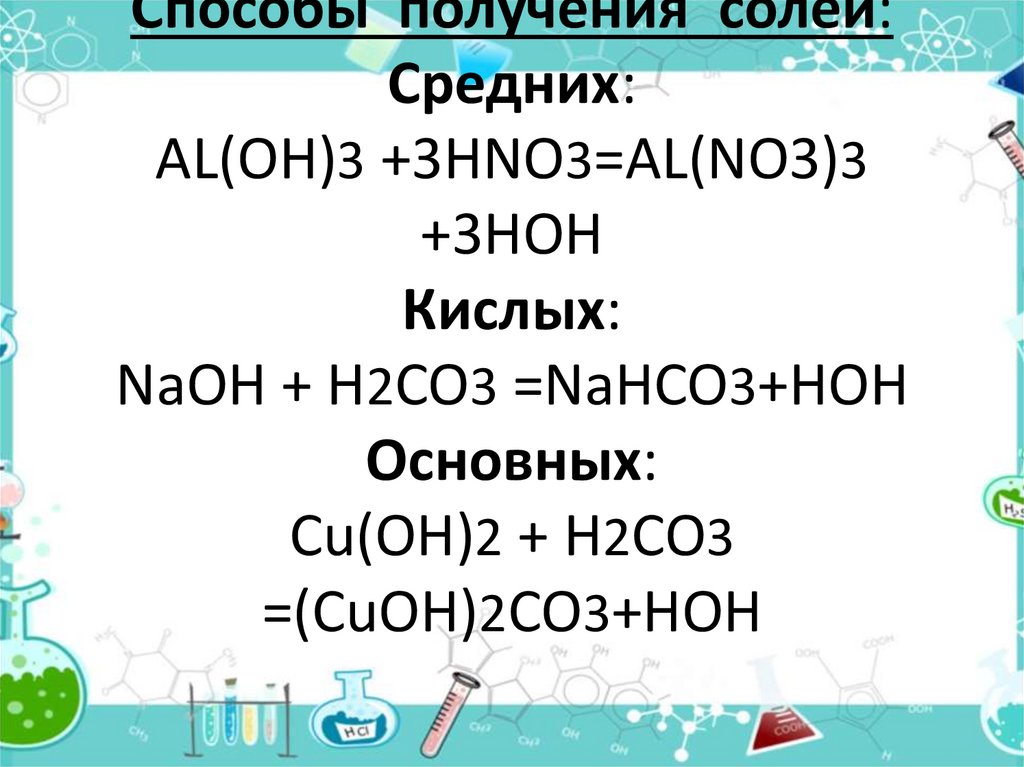
 HNO3 + Na2CO3 --> NaNO3 + NaHCO3 If again The leftover Sodium hydrogen carbonate is made to react with Nitric Acid, then the products will be: HNO3 + NaHCO3 --> NaNo3 + H2O + CO2.
HNO3 + Na2CO3 --> NaNO3 + NaHCO3 If again The leftover Sodium hydrogen carbonate is made to react with Nitric Acid, then the products will be: HNO3 + NaHCO3 --> NaNo3 + H2O + CO2.
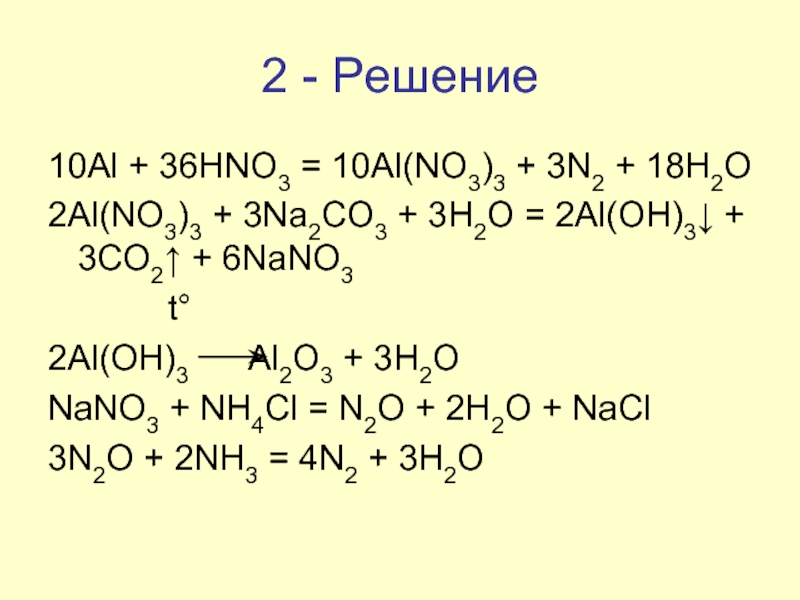
 What is the Ag2CO3 and HNO3 equation? When Ag2CO3 is reacted with HNO3, it forms AgNO3, CO2, and H2O. The balanced chemical equation for this reaction is: 2Ag2CO3 + 2HNO3 → 4AgNO3 + 2CO2 + H2O
What is the Ag2CO3 and HNO3 equation? When Ag2CO3 is reacted with HNO3, it forms AgNO3, CO2, and H2O. The balanced chemical equation for this reaction is: 2Ag2CO3 + 2HNO3 → 4AgNO3 + 2CO2 + H2O
 Wiki User. ∙ 15y ago. Na2CO3 + 2 HNO3 = 2 NaNO3 + CO2 + H2O. the balanced chemical equation of sodium bicarbonate and water making carbonic acid is given as follows.NaHCO3 + H2O NaOH + H2CO3This.
Wiki User. ∙ 15y ago. Na2CO3 + 2 HNO3 = 2 NaNO3 + CO2 + H2O. the balanced chemical equation of sodium bicarbonate and water making carbonic acid is given as follows.NaHCO3 + H2O NaOH + H2CO3This.
 Best Answer. Yellowish white. Wiki User. ∙ 15y ago. More answers. AnswerBot. ∙ 5mo ago. The reaction between AgNO3 and Na2CO3 forms a white precipitate of silver carbonate (Ag2CO3). Wiki User.
Best Answer. Yellowish white. Wiki User. ∙ 15y ago. More answers. AnswerBot. ∙ 5mo ago. The reaction between AgNO3 and Na2CO3 forms a white precipitate of silver carbonate (Ag2CO3). Wiki User.
 HNO3 is an example of a strong acid, while H2CO3 is an example of a weak acid. Both are important in the context of acid-base chemistry and can donate protons in aqueous solutions.
HNO3 is an example of a strong acid, while H2CO3 is an example of a weak acid. Both are important in the context of acid-base chemistry and can donate protons in aqueous solutions.
 The chemical reaction between nitric acid (HNO3) and sodium carbonate (Na2CO3) is: 2 HNO3 + Na2CO3 → 2 NaNO3 + H2O + CO2. In this reaction, nitric acid reacts with sodium carbonate to produce.
The chemical reaction between nitric acid (HNO3) and sodium carbonate (Na2CO3) is: 2 HNO3 + Na2CO3 → 2 NaNO3 + H2O + CO2. In this reaction, nitric acid reacts with sodium carbonate to produce.
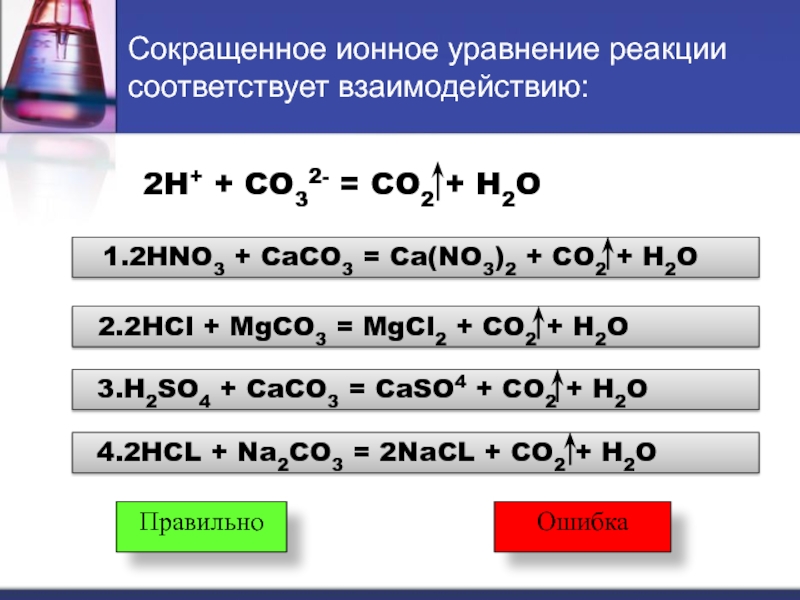
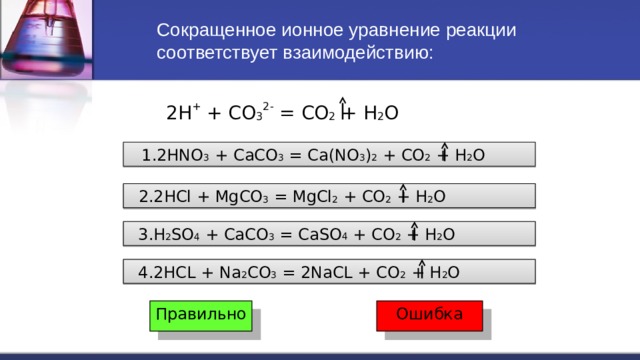
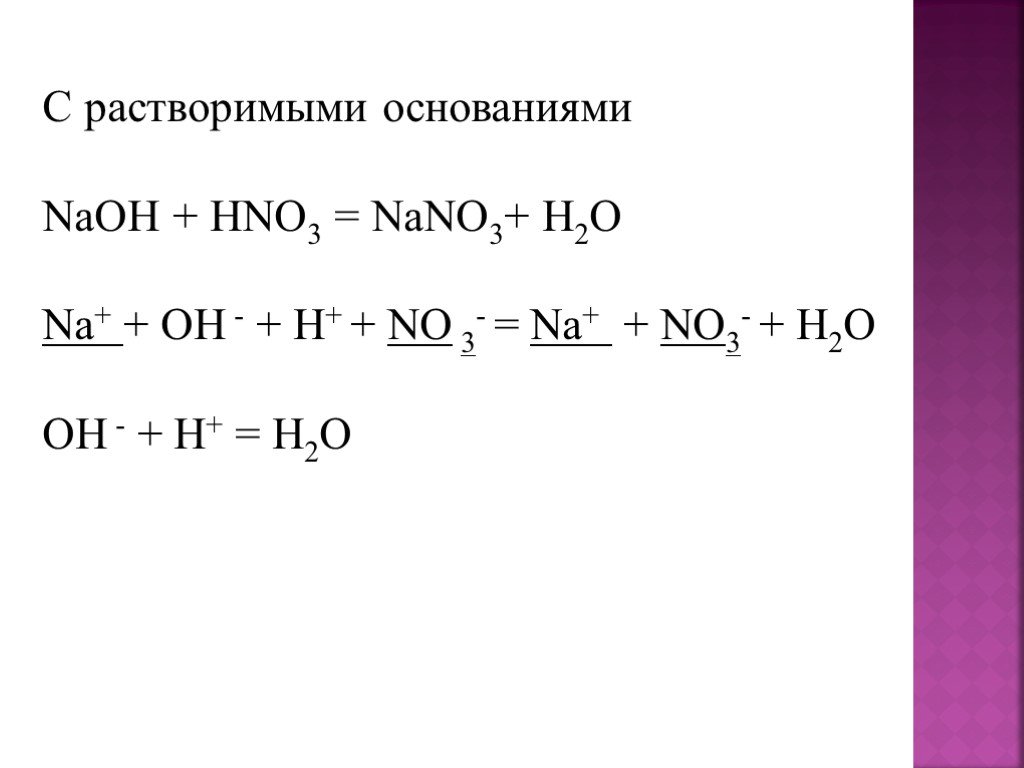
 In the reaction between Na2CO3(aq) and HNO3(aq), the spectator ion is Na+. This ion remains unchanged throughout the reaction and does not participate in forming the products of the reaction.
In the reaction between Na2CO3(aq) and HNO3(aq), the spectator ion is Na+. This ion remains unchanged throughout the reaction and does not participate in forming the products of the reaction.
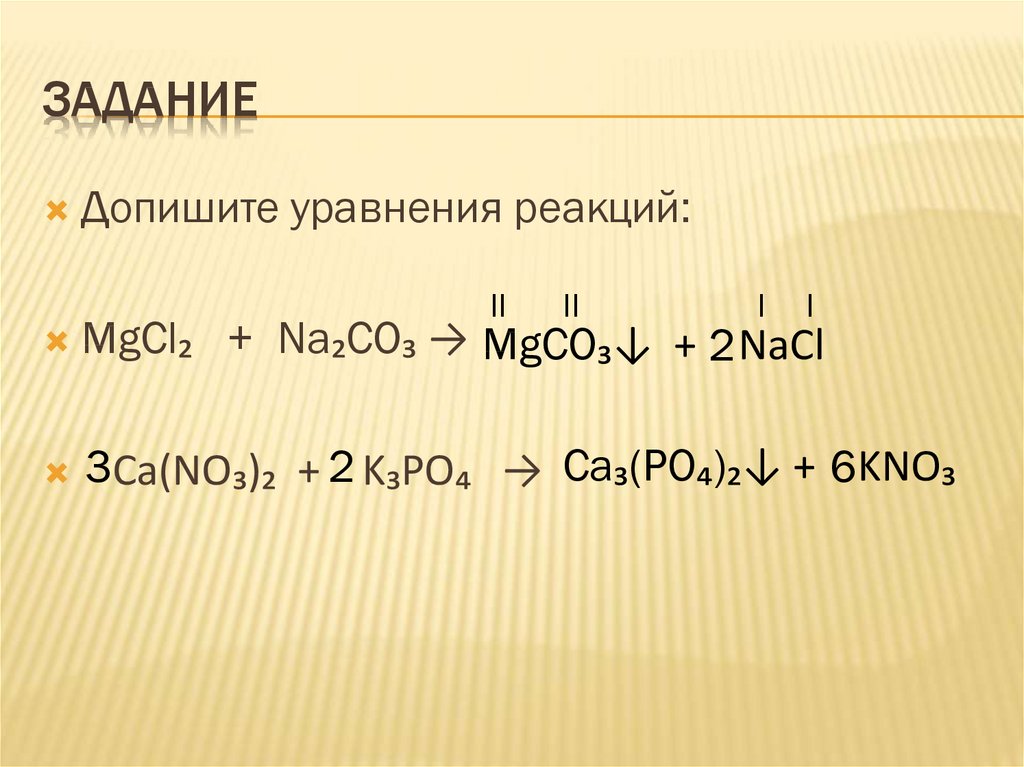
 The balanced equation is: 2 NaHCO3 -> Na2CO3 + H2O + CO2 Therefore, for every 2 moles of NaHCO3, we get 1 mole of NaCl. Therefore, 3.25 moles of NaHCO3 would produce 1.625 moles of NaCl.
The balanced equation is: 2 NaHCO3 -> Na2CO3 + H2O + CO2 Therefore, for every 2 moles of NaHCO3, we get 1 mole of NaCl. Therefore, 3.25 moles of NaHCO3 would produce 1.625 moles of NaCl.
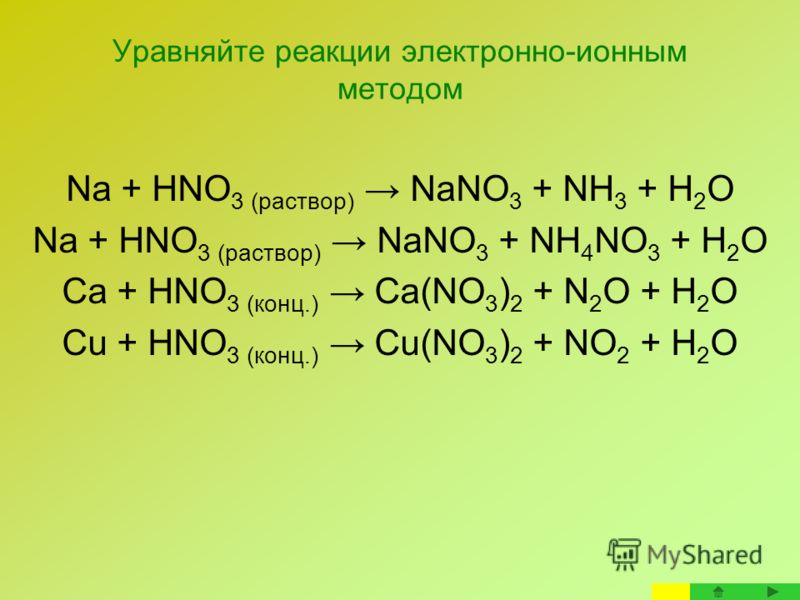
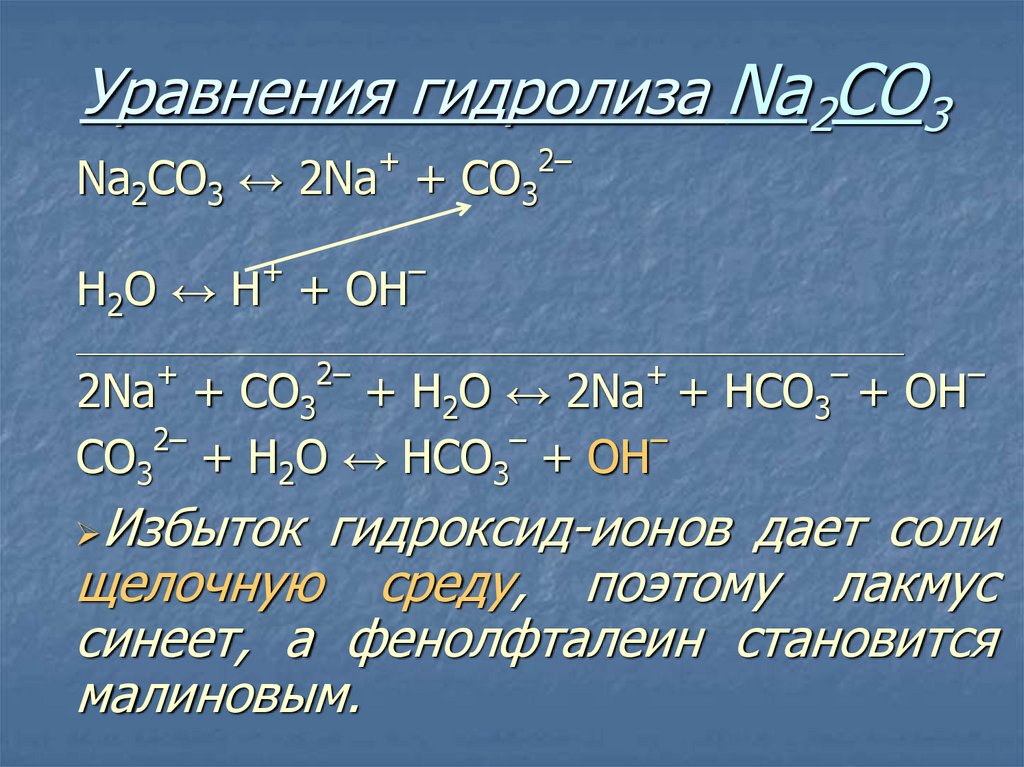

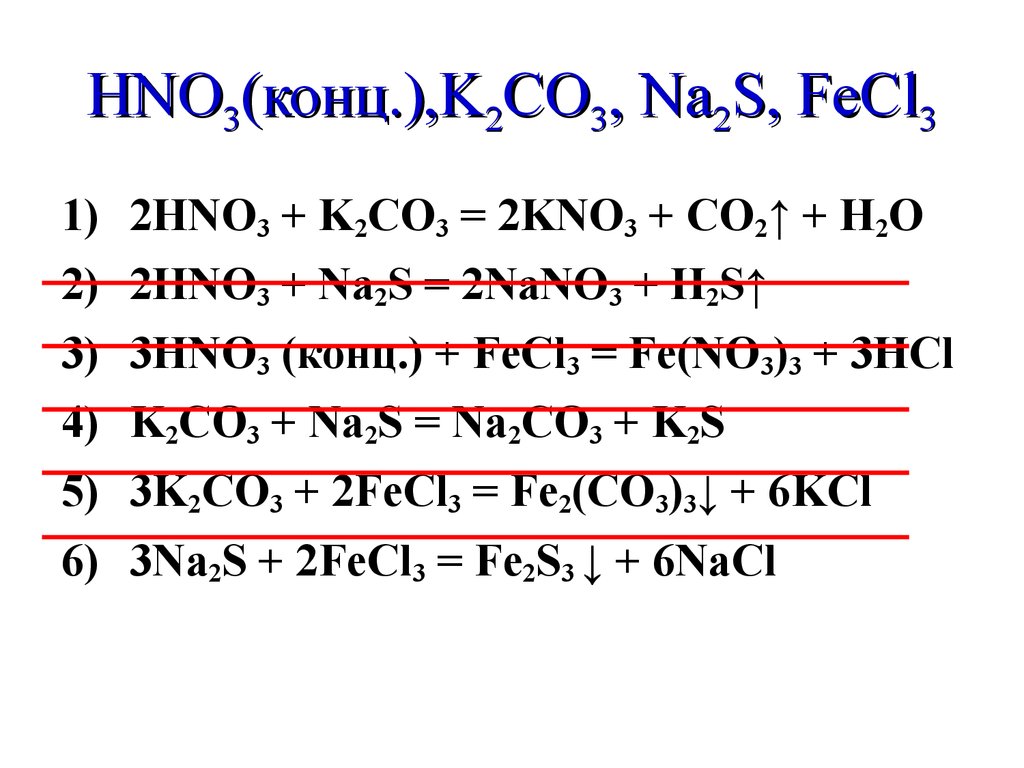
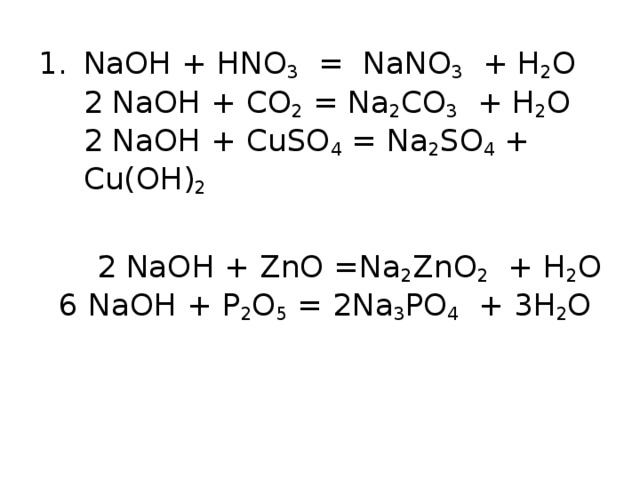
Еще по теме:








Еще по теме: