 Сульфат натрия, сернокислый натрий, Na2SO4 — натриевая соль серной кислоты. Это бесцветные кристаллы, хорошо растворяющиеся в воде, образует кристаллогидраты, самый известный из них — декагидрат, получивший традиционное название — «глауберова соль». Обладает … See more
Сульфат натрия, сернокислый натрий, Na2SO4 — натриевая соль серной кислоты. Это бесцветные кристаллы, хорошо растворяющиеся в воде, образует кристаллогидраты, самый известный из них — декагидрат, получивший традиционное название — «глауберова соль». Обладает … See more
 Хлорид натрия (NaCl) и сульфат натрия (Na2SO4) - распространенные соли, которые широко используются в быту, промышленности, медицине. Но мало кто знает об удивительных …
Хлорид натрия (NaCl) и сульфат натрия (Na2SO4) - распространенные соли, которые широко используются в быту, промышленности, медицине. Но мало кто знает об удивительных …
 Химические Уравнения oнлайн! Приложение для вычисления и дополнения продуктов реакции. Ионные и окислительно-восстановительные реакции!
Химические Уравнения oнлайн! Приложение для вычисления и дополнения продуктов реакции. Ионные и окислительно-восстановительные реакции!
 As you know, sodium sulfate ,"Na"_2"SO"_4, and sodium chloride, "NaCl", are both ionic compounds that are soluble in aqueous solution. This means that in a solution of …
As you know, sodium sulfate ,"Na"_2"SO"_4, and sodium chloride, "NaCl", are both ionic compounds that are soluble in aqueous solution. This means that in a solution of …
 In this paper, we present development of a comprehensive engineering thermodynamic model for the binary systems of NaCl + H 2 O and Na 2 SO 4 + H 2 O and the …
In this paper, we present development of a comprehensive engineering thermodynamic model for the binary systems of NaCl + H 2 O and Na 2 SO 4 + H 2 O and the …
 In this study, NaCl and Na 2 SO 4 were taken as examples and mixed in the solution. It was found that the agglomeration of the crystals occurred both in the drying and …
In this study, NaCl and Na 2 SO 4 were taken as examples and mixed in the solution. It was found that the agglomeration of the crystals occurred both in the drying and …
 Chemical properties. Sodium sulfate is a typical electrostatically bonded ionic sulfate. The existence of free sulfate ions in solution is indicated by the easy formation of insoluble sulfates …
Chemical properties. Sodium sulfate is a typical electrostatically bonded ionic sulfate. The existence of free sulfate ions in solution is indicated by the easy formation of insoluble sulfates …
 The present work reports on a simple chemical vapor deposition (CVD) technique that employs alkali halide (NaCl) to synthesize high-quality few-layer MoS2 by reducing growth temperature from 850.
The present work reports on a simple chemical vapor deposition (CVD) technique that employs alkali halide (NaCl) to synthesize high-quality few-layer MoS2 by reducing growth temperature from 850.
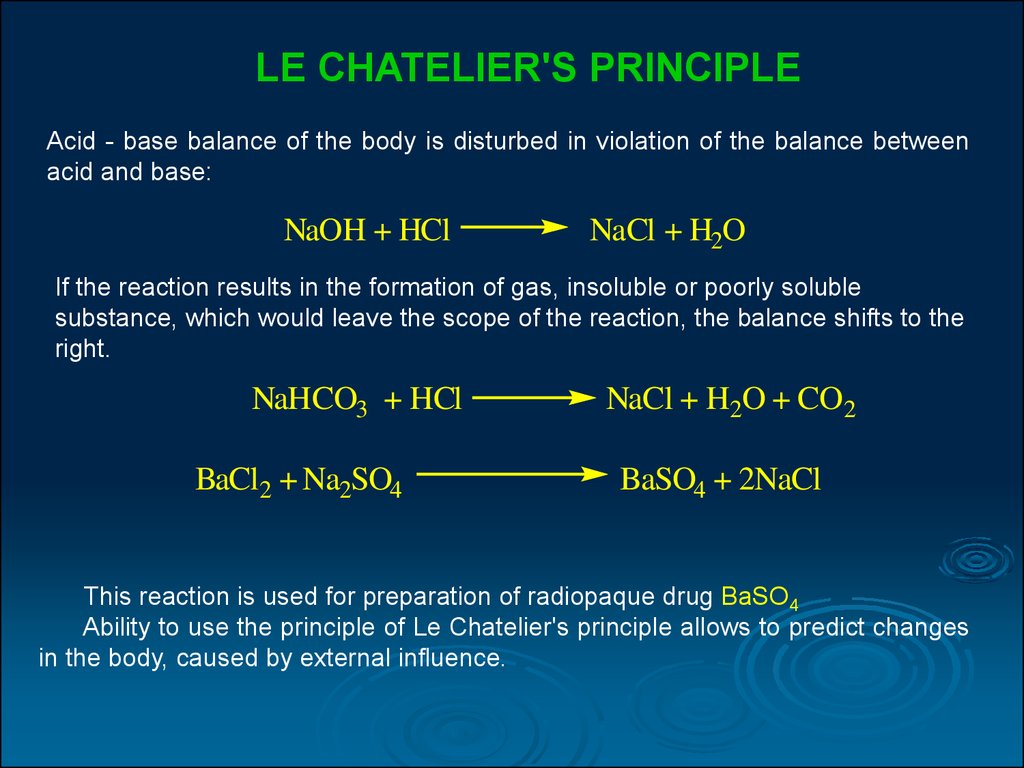
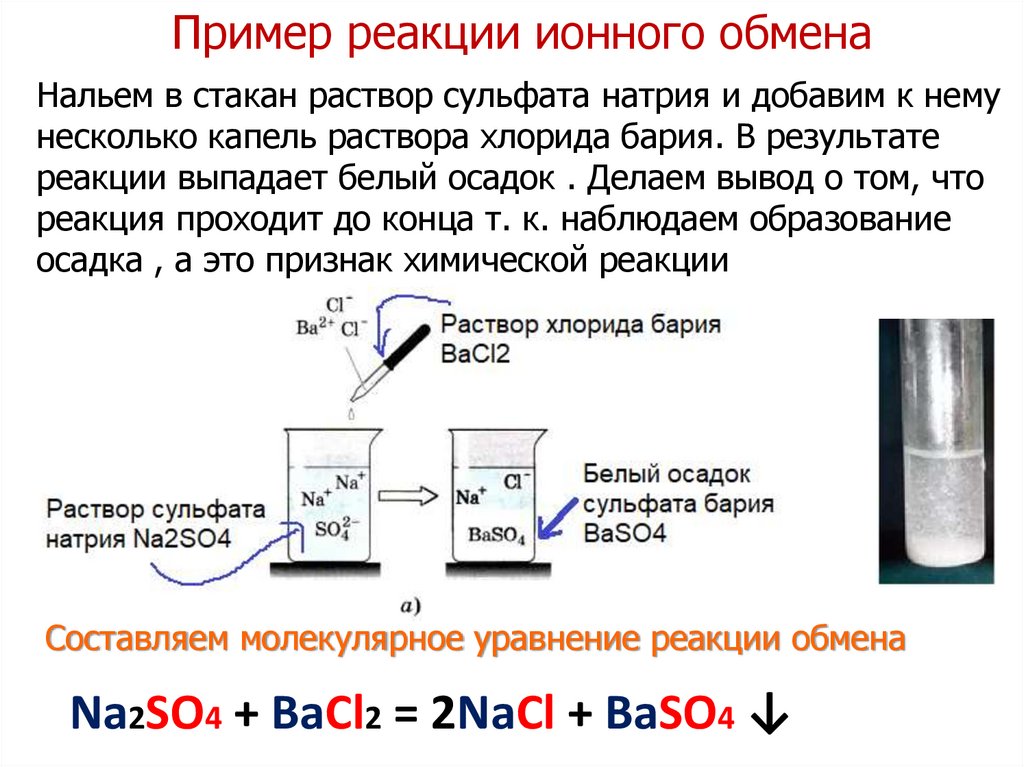
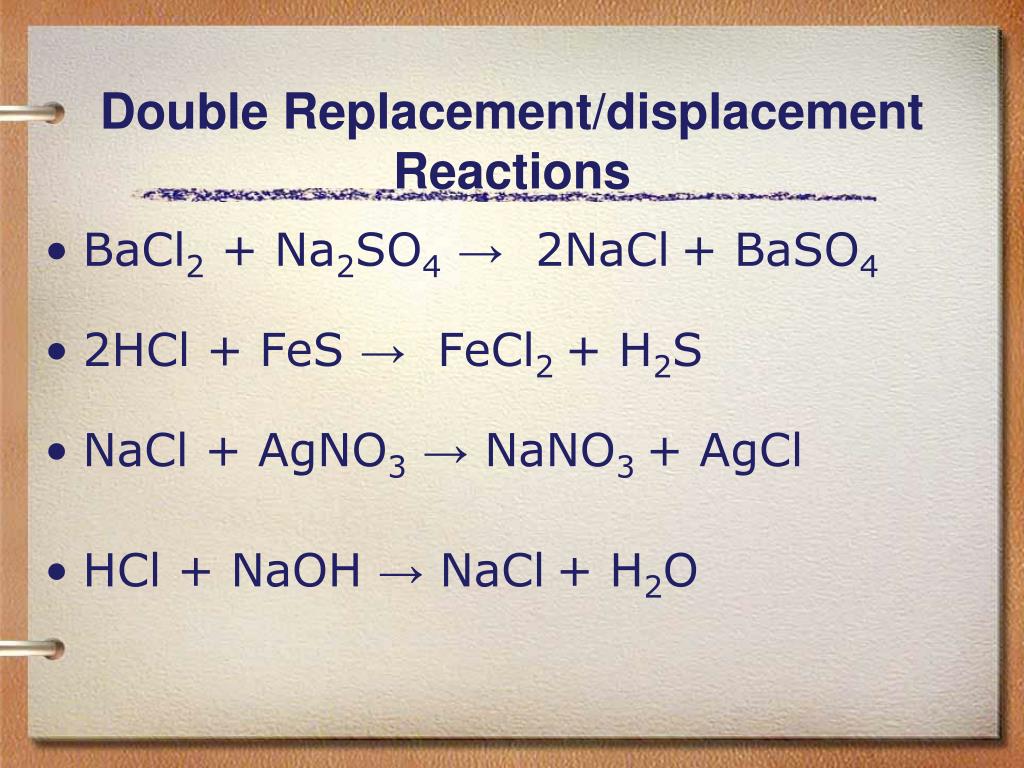
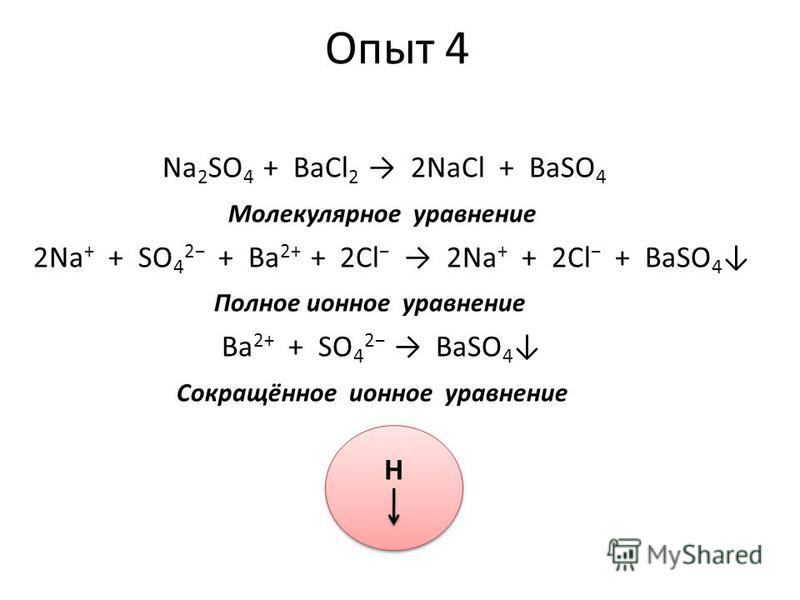
 1 BaCl 2 + 1 Na 2 SO 4 = 1 BaSO 4 + 1 NaCl. For each element, we check if the number of atoms is balanced on both sides of the equation. Ba is balanced: 1 atom in reagents and 1 atom in …
1 BaCl 2 + 1 Na 2 SO 4 = 1 BaSO 4 + 1 NaCl. For each element, we check if the number of atoms is balanced on both sides of the equation. Ba is balanced: 1 atom in reagents and 1 atom in …
 High salinity brine is the main resource for NaCl and Na 2 SO 4. An industrial production test and application for Na 2 CO 3 -NaOH brine purification methods were carried …
High salinity brine is the main resource for NaCl and Na 2 SO 4. An industrial production test and application for Na 2 CO 3 -NaOH brine purification methods were carried …
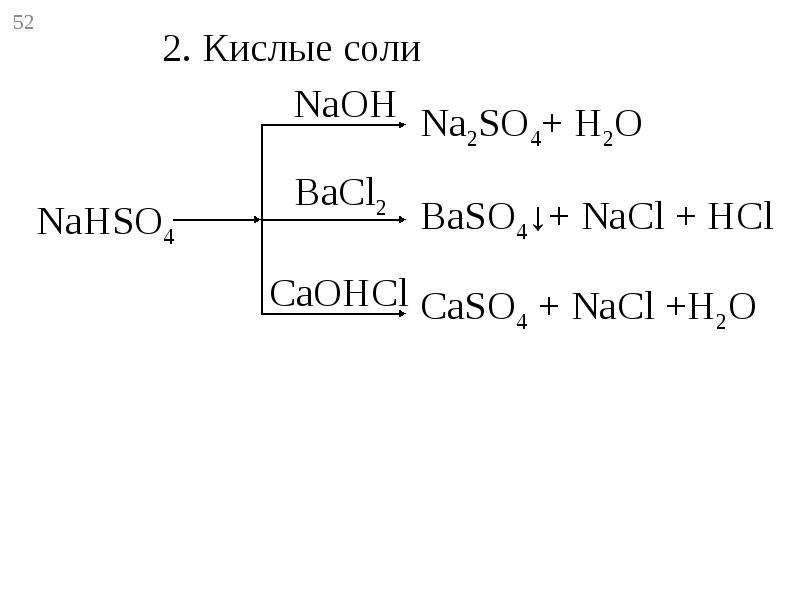 Employing the phase diagram, this study focuses on the crystallization behavior and damage potential of the Na 2 SO 4 –NaCl–H 2 O system during evaporation, by using two …
Employing the phase diagram, this study focuses on the crystallization behavior and damage potential of the Na 2 SO 4 –NaCl–H 2 O system during evaporation, by using two …
 Dual, aqueous solubility behavior of Na2SO4 as a function of temperature is still a natural enigma lying unresolved in literature. The solubility of Na2SO4 increases up to 32.38oC and decreases.
Dual, aqueous solubility behavior of Na2SO4 as a function of temperature is still a natural enigma lying unresolved in literature. The solubility of Na2SO4 increases up to 32.38oC and decreases.
 The Gibbs energy of formation, enthalpy of formation and heat capacity parameters for solids NaCl (s), NaCl·2H 2 O (s), Na 2 SO 4(s) and Na 2 SO 4 ·10H 2 O (s) are obtained by …
The Gibbs energy of formation, enthalpy of formation and heat capacity parameters for solids NaCl (s), NaCl·2H 2 O (s), Na 2 SO 4(s) and Na 2 SO 4 ·10H 2 O (s) are obtained by …
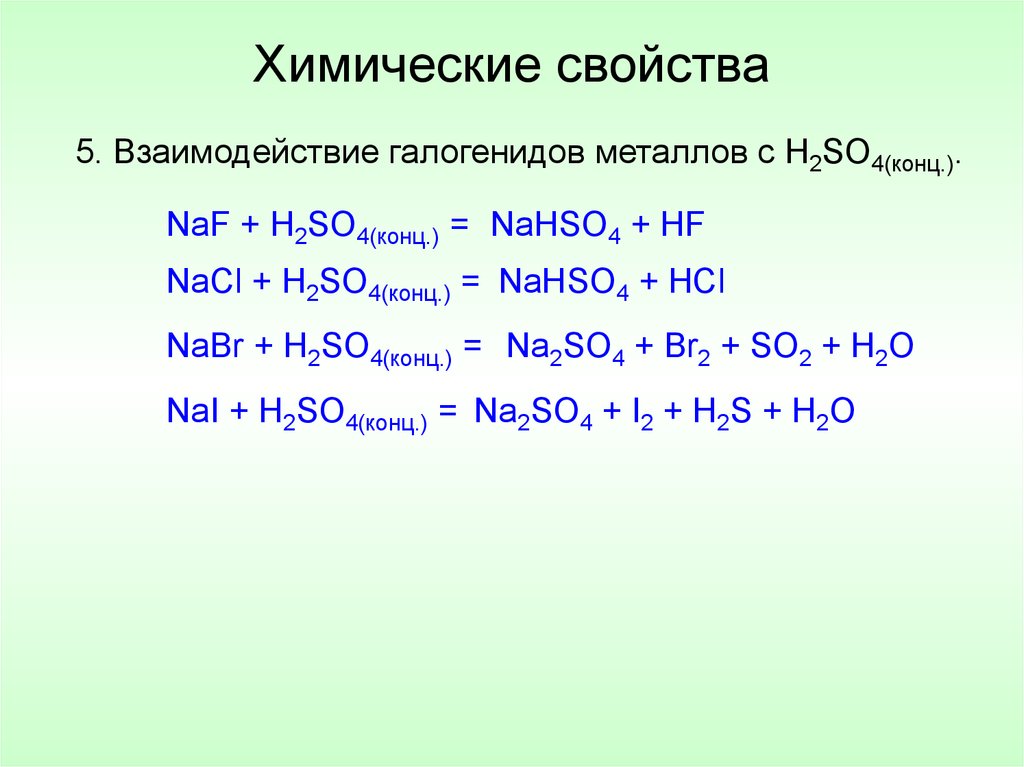
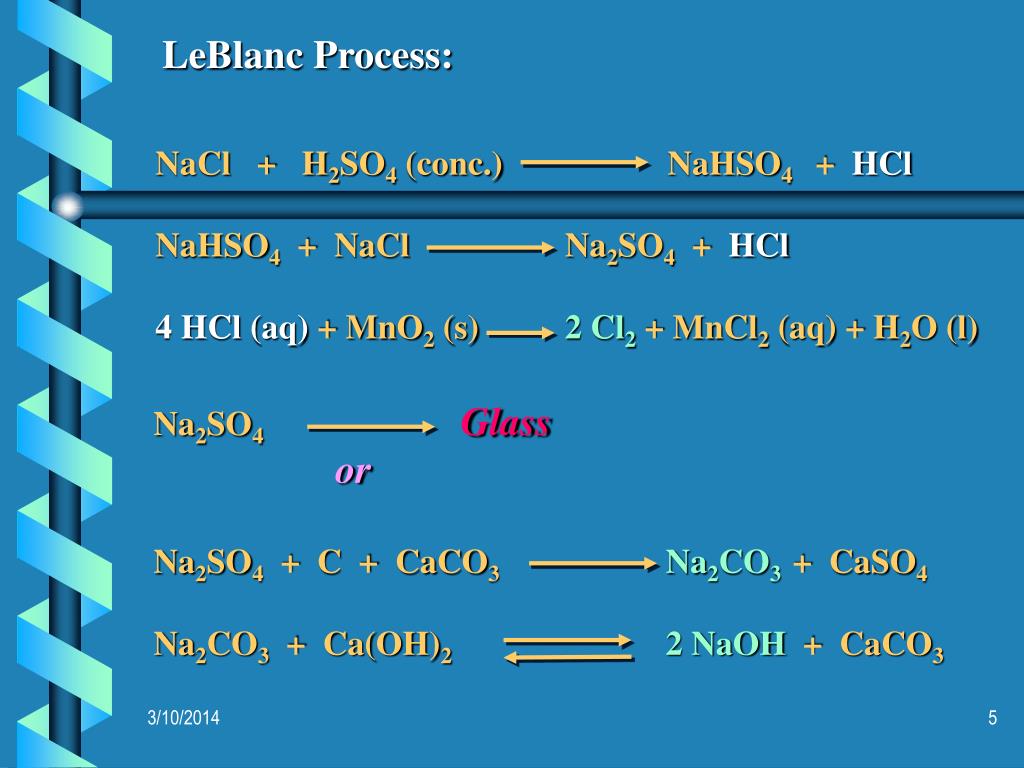
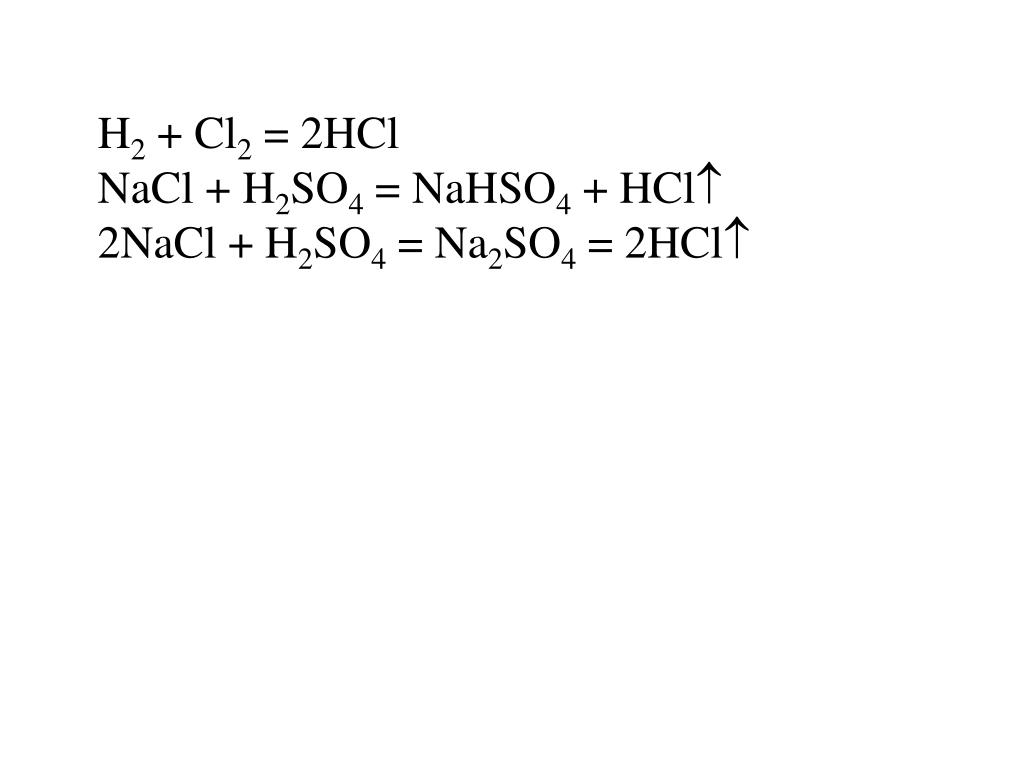 Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 NaCl + 1 H 2 SO 4 = 1 Na 2 SO 4 + 1 HCl. For each element, we check if the number of atoms is …
Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 NaCl + 1 H 2 SO 4 = 1 Na 2 SO 4 + 1 HCl. For each element, we check if the number of atoms is …
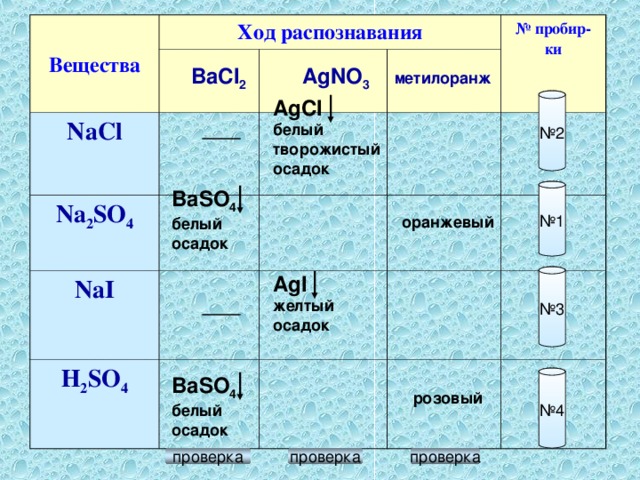
Еще по теме:






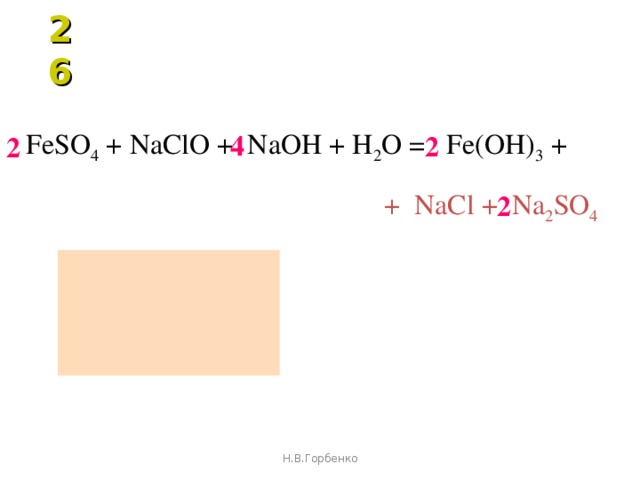


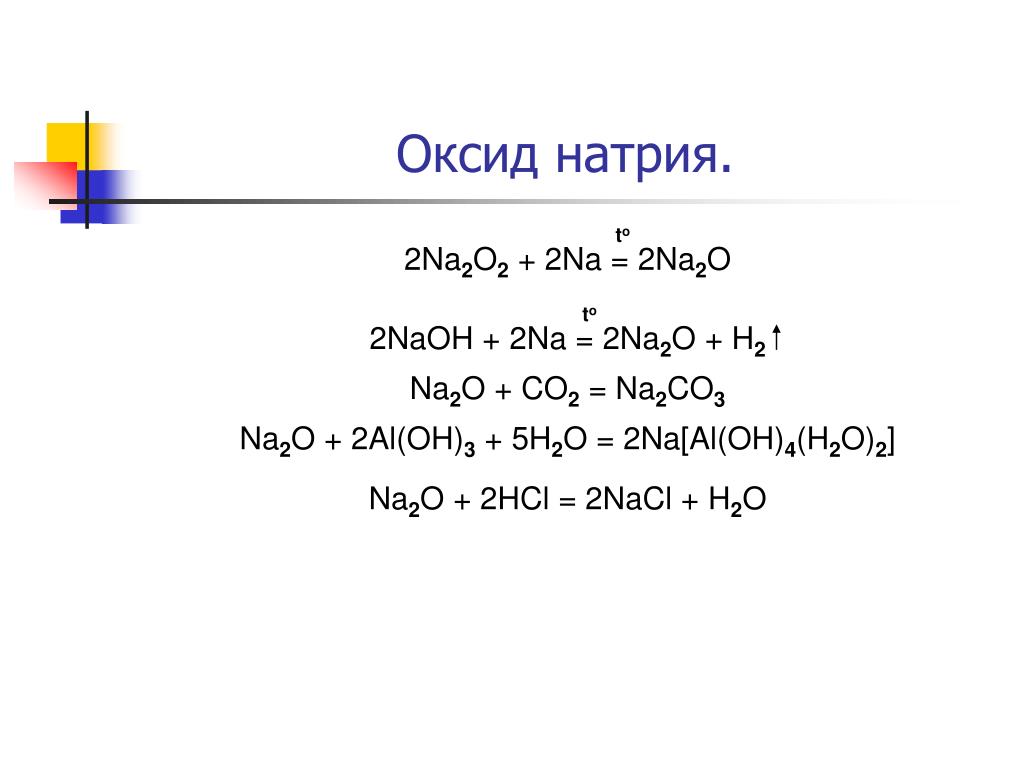
Еще по теме: