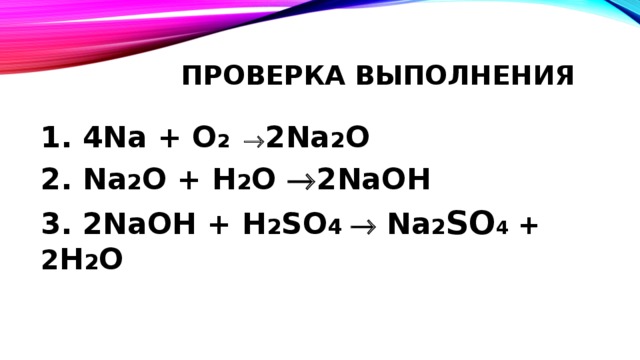
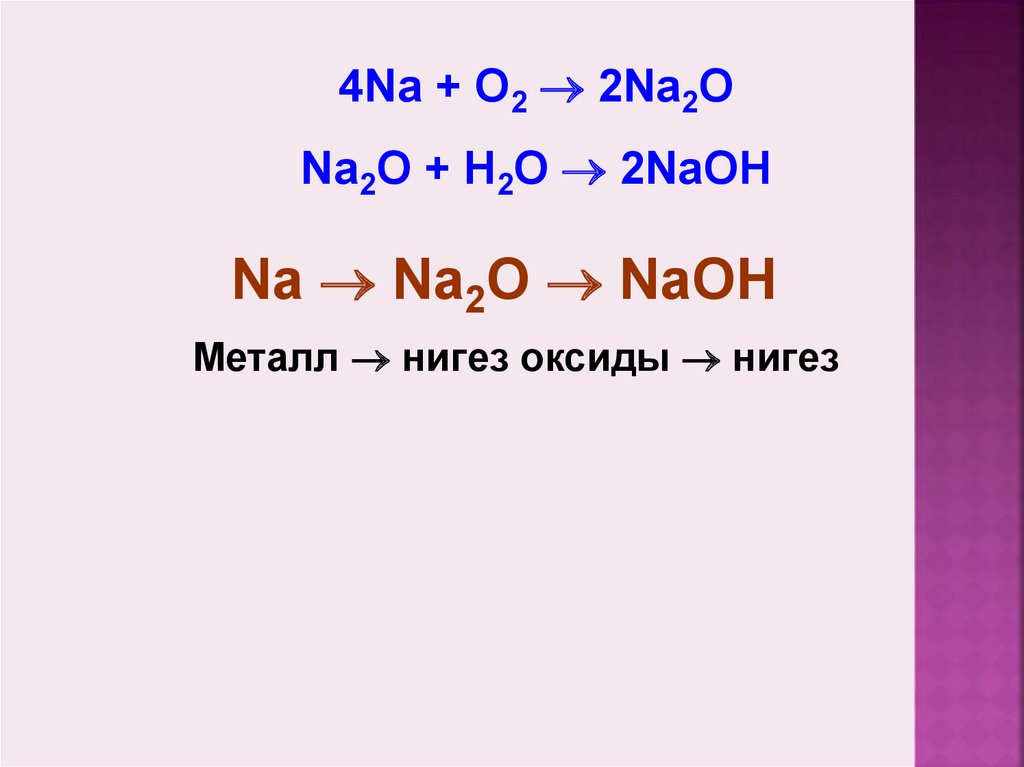
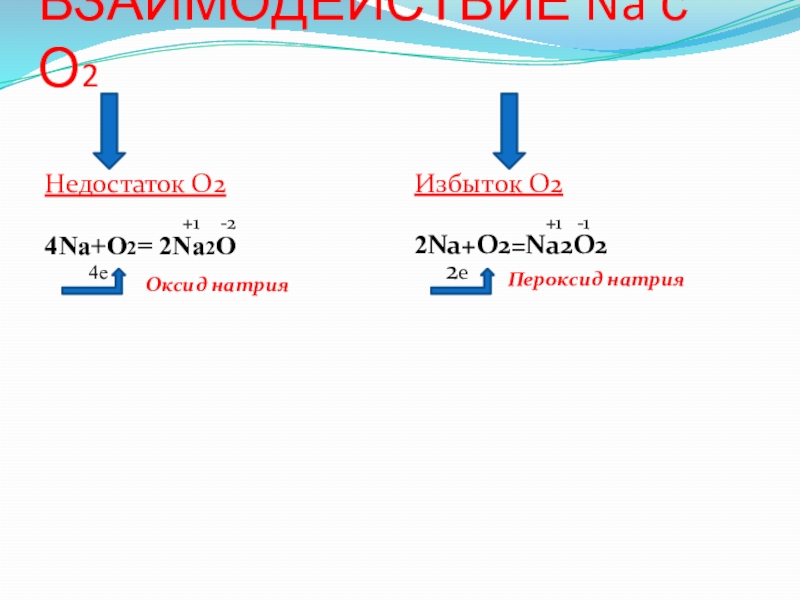
Решенное и коэффициентами уравнение реакции 4 Na + O2 → 2 Na2O с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 4 Na + O2 → 2 Na2O с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
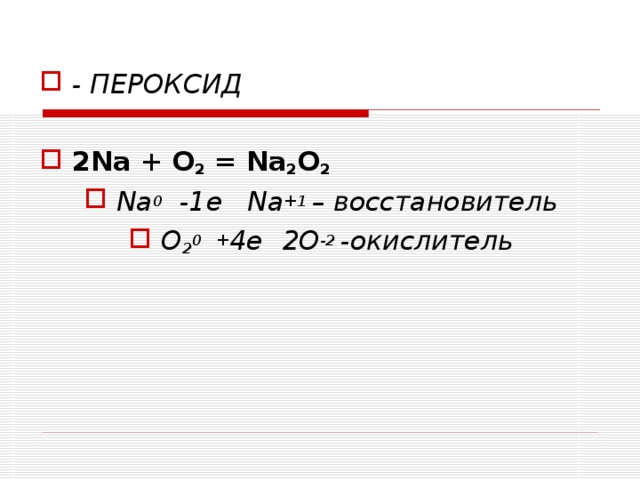
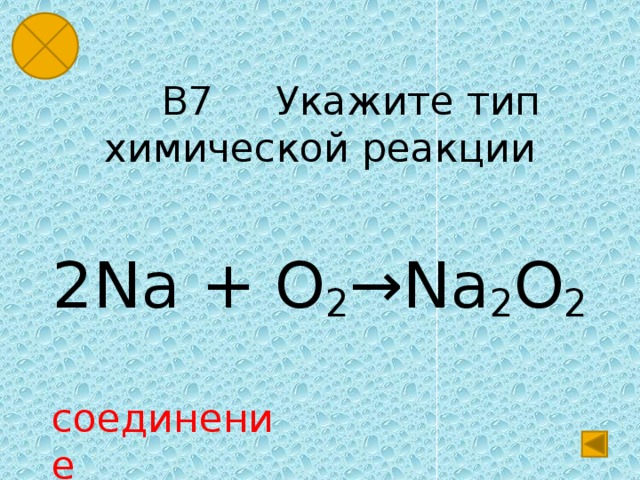
 Решенное и коэффициентами уравнение реакции 2 Na + O2 → Na2O2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 Na + O2 → Na2O2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Na + O2 = Na2O is a Synthesis reaction where four moles of Sodium [Na] and one mole of Dioxygen [O 2] combine to form two moles of Sodium Oxide [Na 2 O]
Na + O2 = Na2O is a Synthesis reaction where four moles of Sodium [Na] and one mole of Dioxygen [O 2] combine to form two moles of Sodium Oxide [Na 2 O]
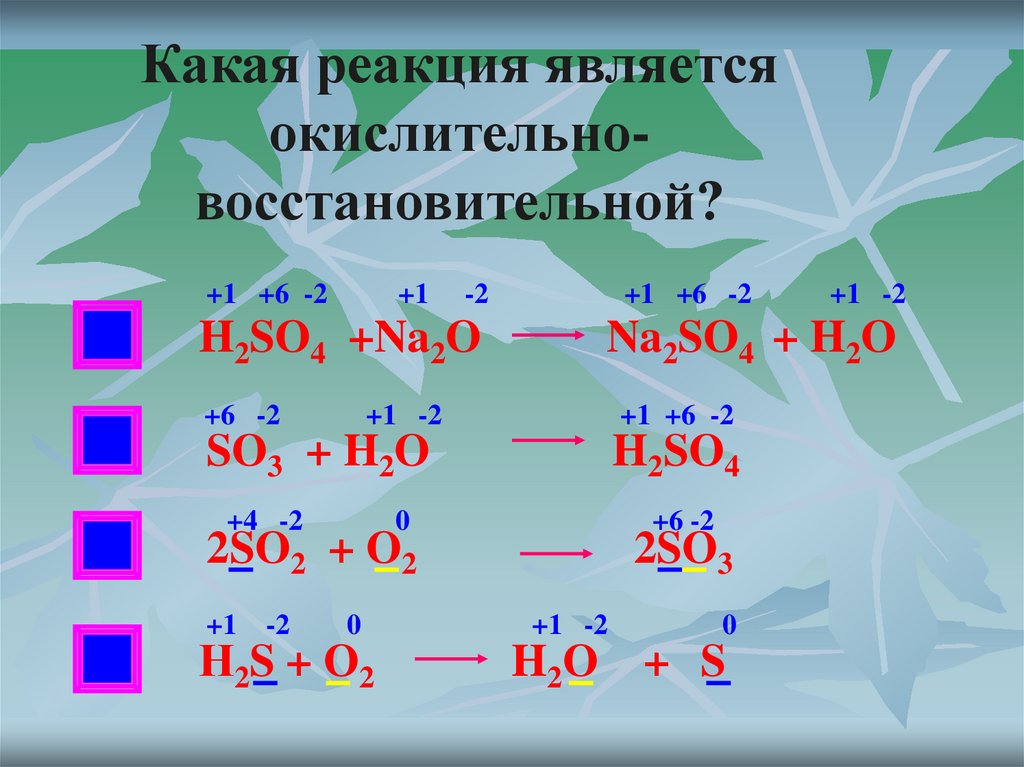
 For example, in the reaction of hydrogen (H₂) with oxygen (O₂) to form water (H₂O), the chemical equation is: H 2 + O 2 = H 2 O However, this equation isn't balanced because the number of …
For example, in the reaction of hydrogen (H₂) with oxygen (O₂) to form water (H₂O), the chemical equation is: H 2 + O 2 = H 2 O However, this equation isn't balanced because the number of …
 Na + O2 = Na2O + Na2O2 is a Double Displacement (Metathesis) reaction where eight moles of Sodium [Na] and three moles of Dioxygen [O 2] react to form two moles of Sodium Oxide [Na …
Na + O2 = Na2O + Na2O2 is a Double Displacement (Metathesis) reaction where eight moles of Sodium [Na] and three moles of Dioxygen [O 2] react to form two moles of Sodium Oxide [Na …
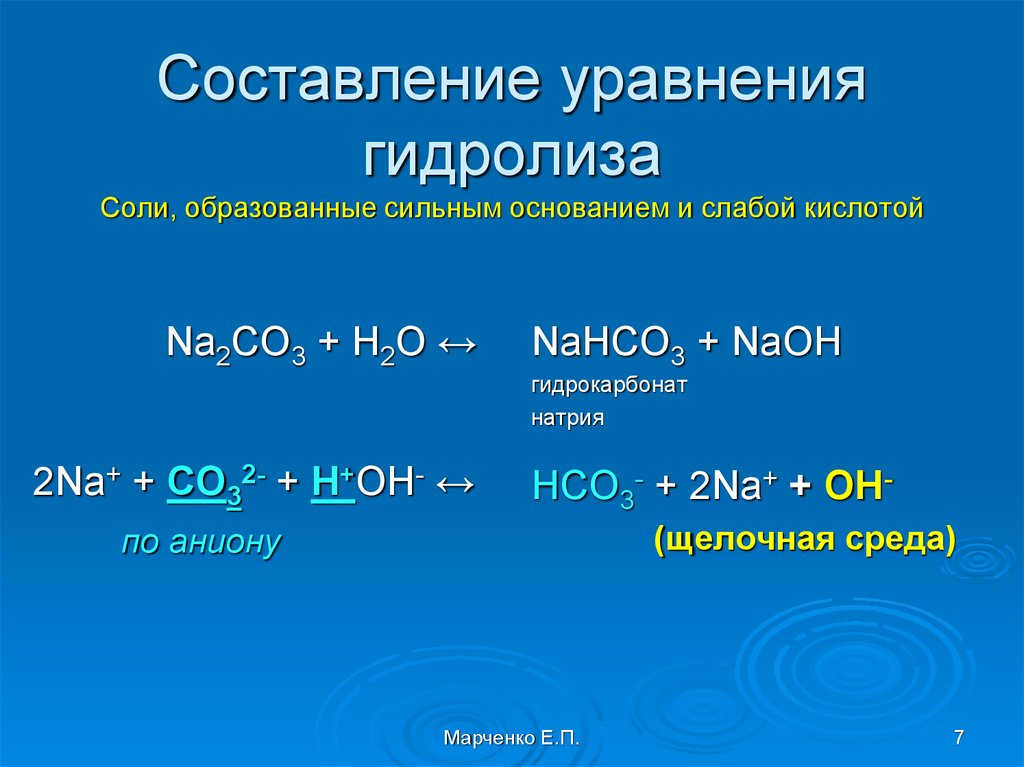
 To balance Na2O + O2 = Na2O2 you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change the.
To balance Na2O + O2 = Na2O2 you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change the.
 In this video we'll balance the equation Na + O2 = Na2O2 and provide the correct coefficients for each compound.To balance Na + O2 = Na2O2 you'll need to be.
In this video we'll balance the equation Na + O2 = Na2O2 and provide the correct coefficients for each compound.To balance Na + O2 = Na2O2 you'll need to be.
 Na2O2 = Na2O + O2 is a Decomposition reaction where two moles of Sodium Peroxide [Na 2 O 2] decomposes into two moles of Sodium Oxide [Na 2 O] and one mole of Dioxygen [O 2]
Na2O2 = Na2O + O2 is a Decomposition reaction where two moles of Sodium Peroxide [Na 2 O 2] decomposes into two moles of Sodium Oxide [Na 2 O] and one mole of Dioxygen [O 2]
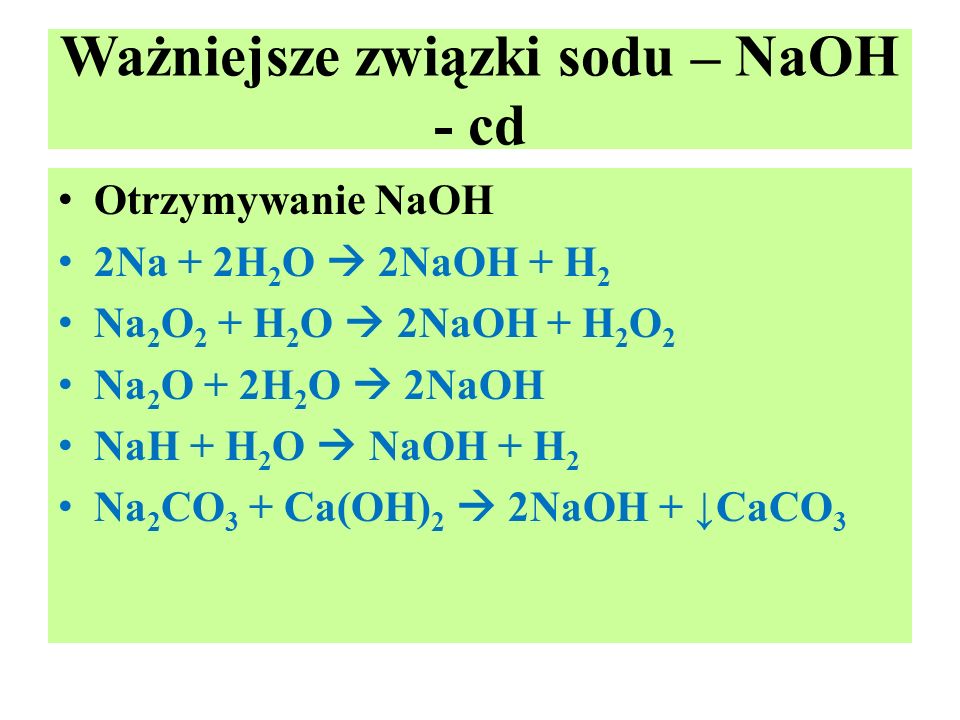
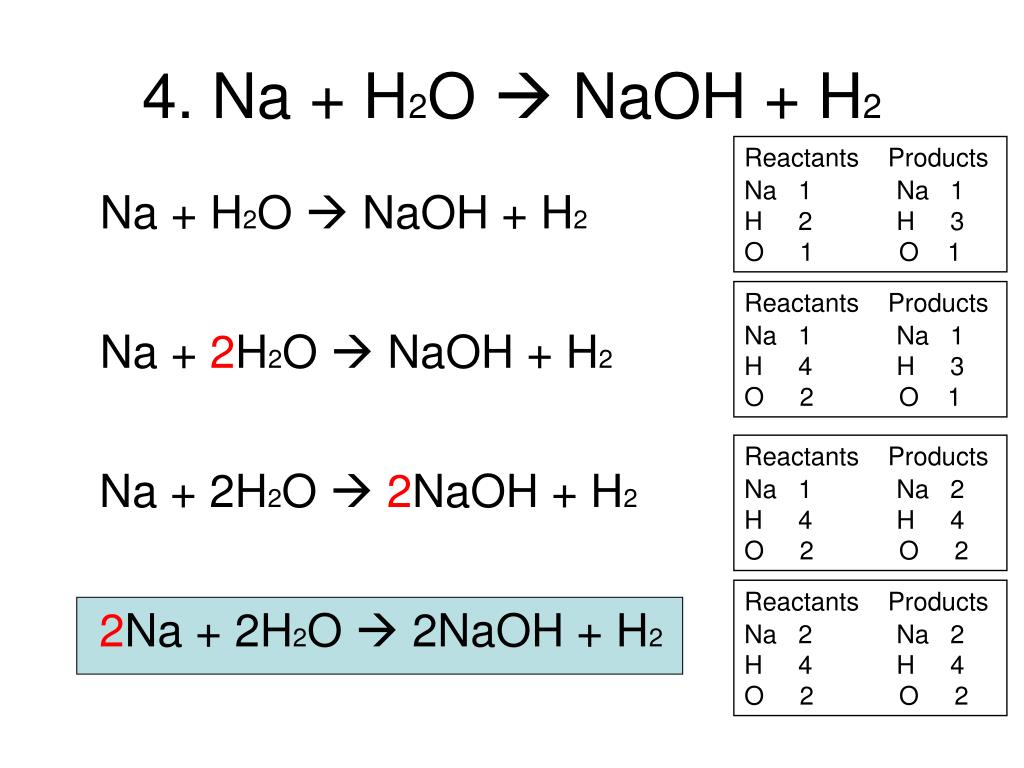
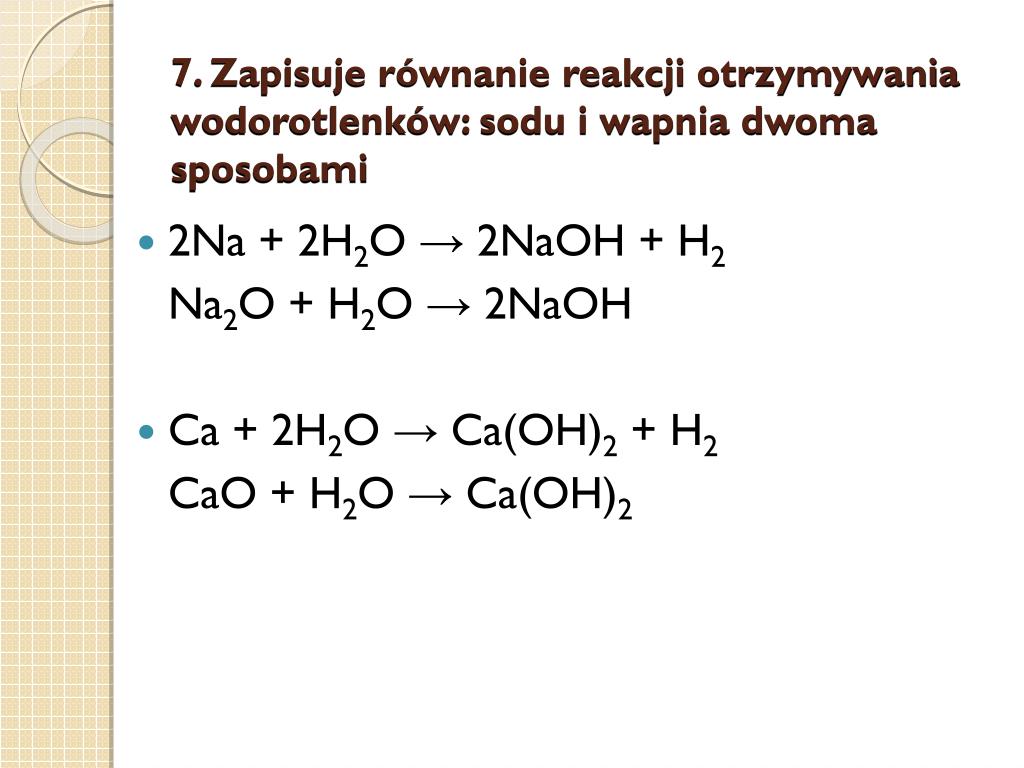
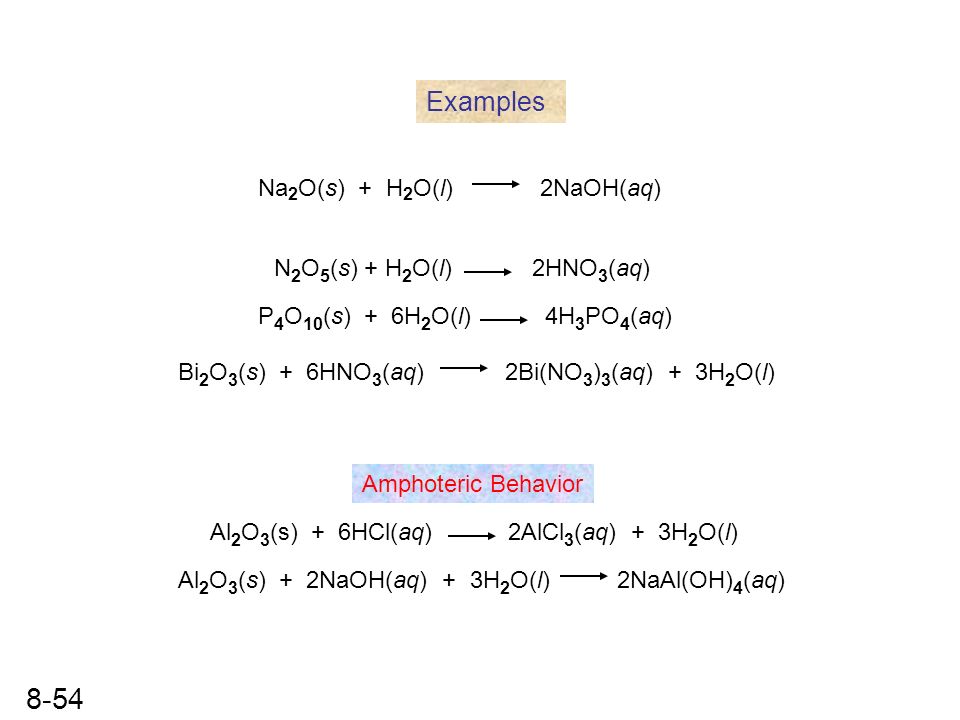
 4Na + O2 → 2Na2O. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of …
4Na + O2 → 2Na2O. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of …
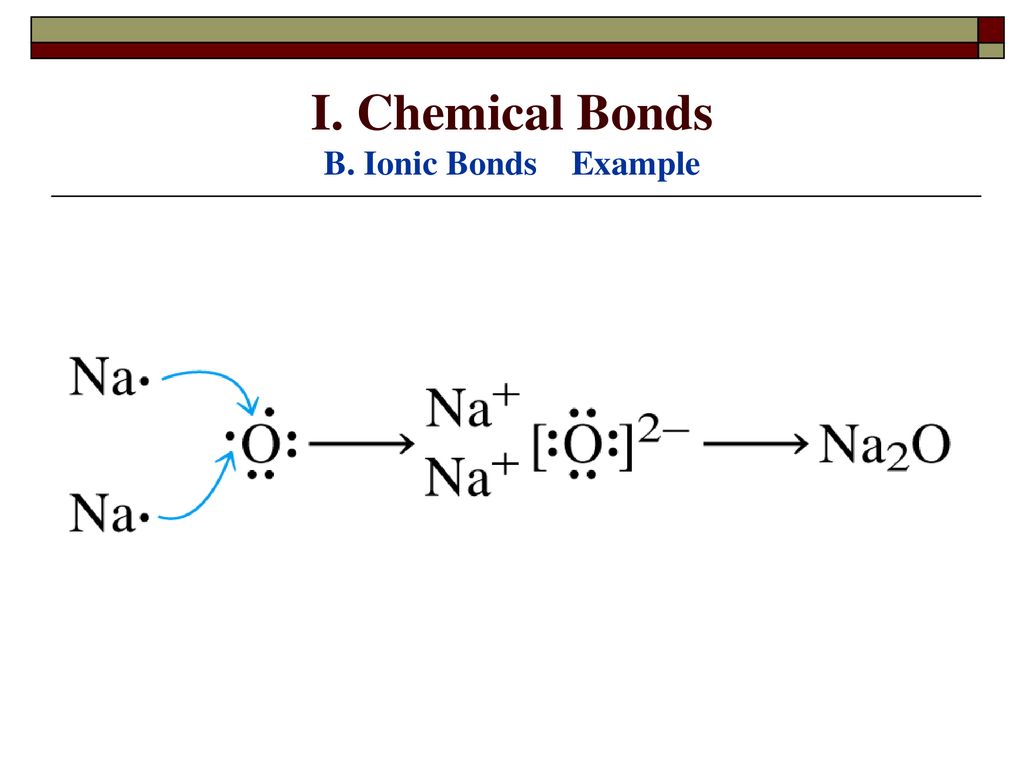
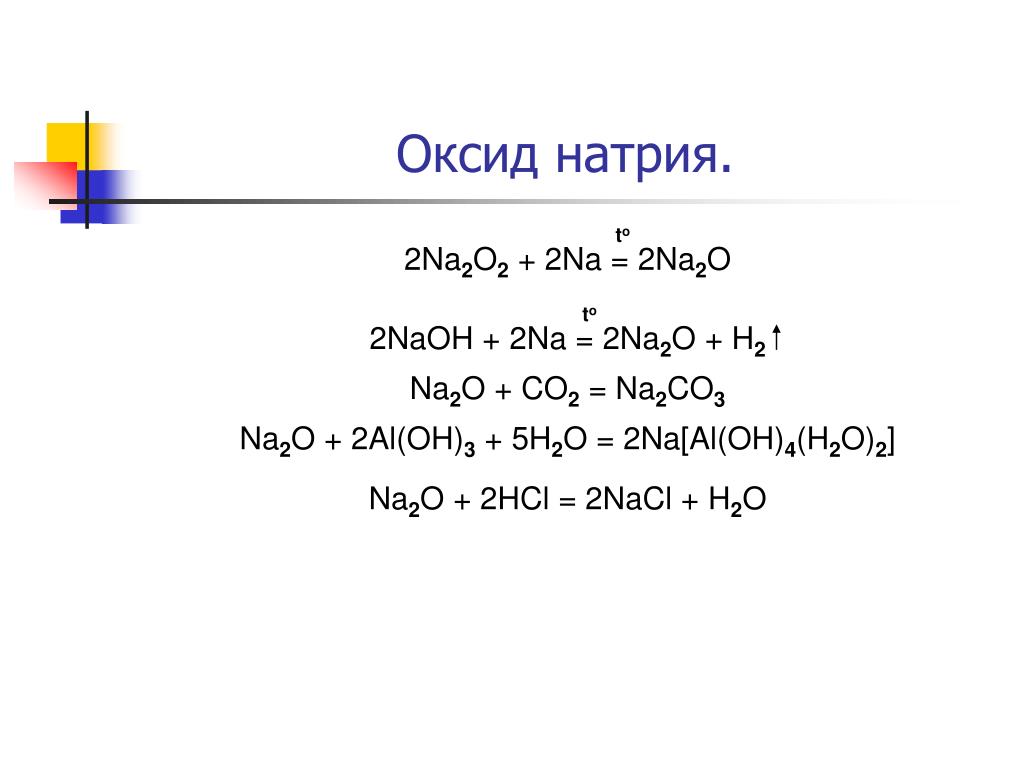
 Na 2 O is a sodium oxide constituted by the chemical reaction between sodium metallic element and chemical element of oxygen in the air at room temperature.
Na 2 O is a sodium oxide constituted by the chemical reaction between sodium metallic element and chemical element of oxygen in the air at room temperature.
 To balance Na2O2 = Na2O + O2 you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change the.
To balance Na2O2 = Na2O + O2 you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change the.
 Then for balancing the Na atoms, add a stoichiometric coefficient of 4 on the reactant side and it will balance the whole reaction given by; 4 Na (s) Sodium + O 2 (g Oxygen) → 2 Na 2 O …
Then for balancing the Na atoms, add a stoichiometric coefficient of 4 on the reactant side and it will balance the whole reaction given by; 4 Na (s) Sodium + O 2 (g Oxygen) → 2 Na 2 O …
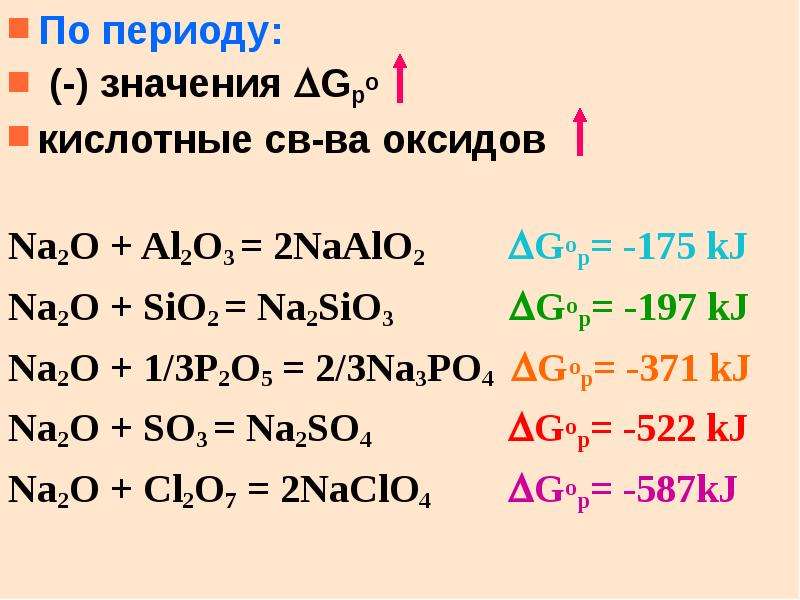
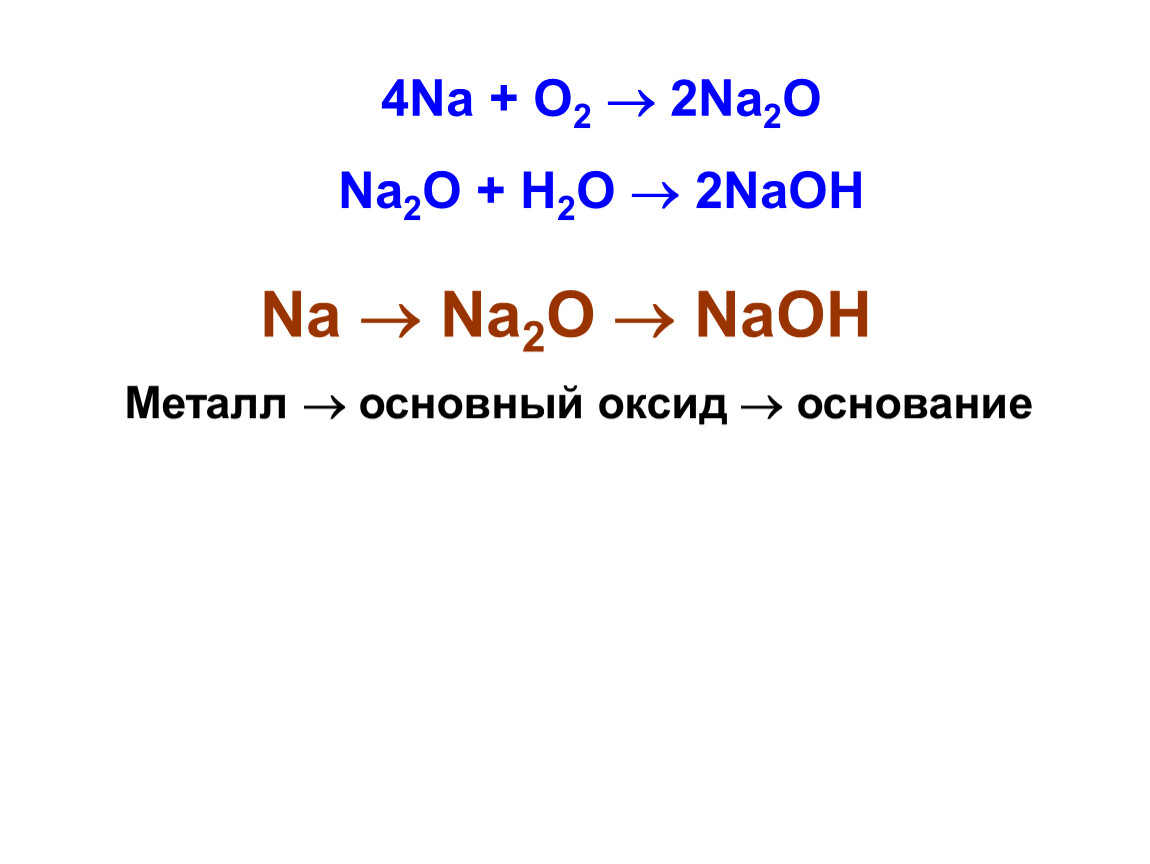 Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to …
Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to …
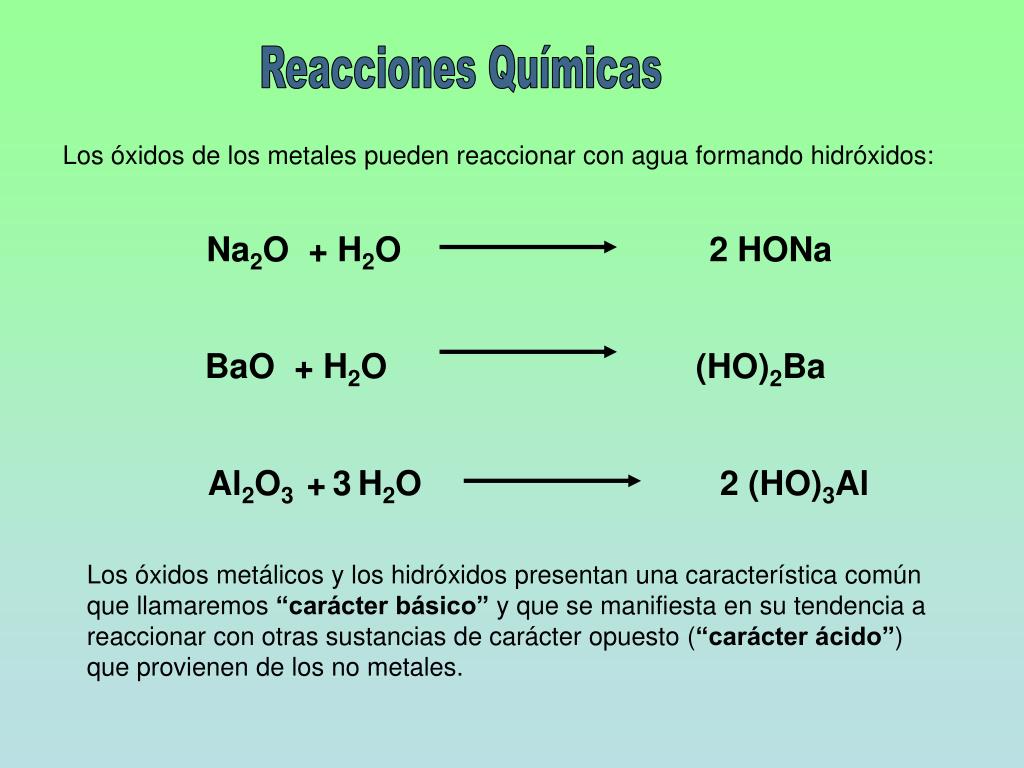
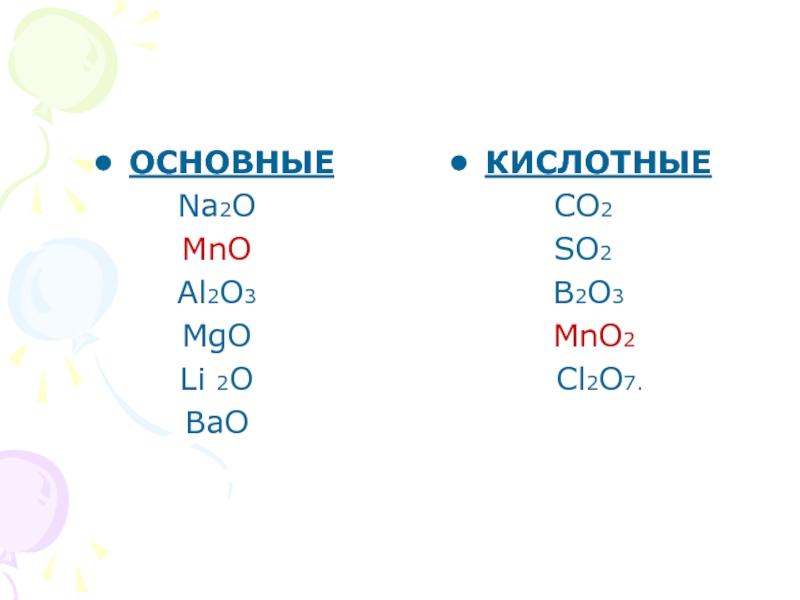 Sodium and Oxygen can combine in a SYNTHESIS reaction to form a simple binary ionic compound, Na2O, sodium oxide. BUT did you know that this only occurs to about 80% of the …
Sodium and Oxygen can combine in a SYNTHESIS reaction to form a simple binary ionic compound, Na2O, sodium oxide. BUT did you know that this only occurs to about 80% of the …
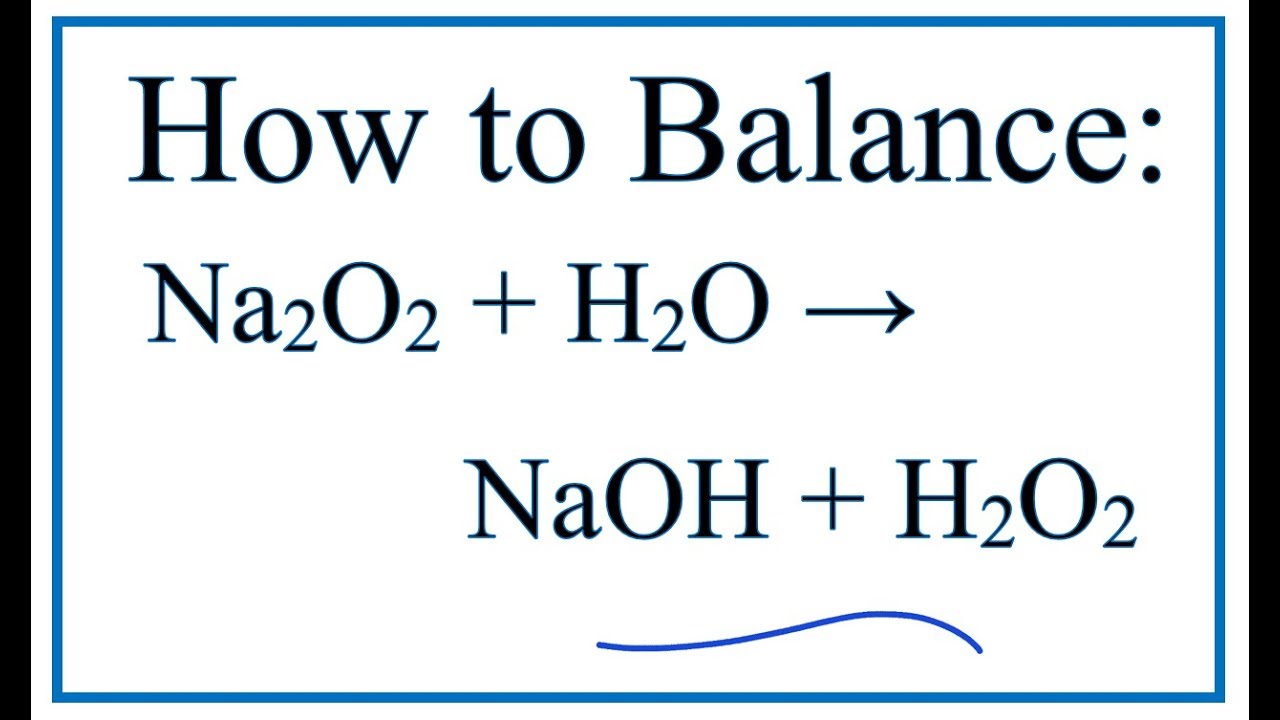 Sodium Oxide is a chemical compound containing sodium and oxygen. It forms when sodium reacts with oxygen, creating a solid substance. The chemical formula of sodium …
Sodium Oxide is a chemical compound containing sodium and oxygen. It forms when sodium reacts with oxygen, creating a solid substance. The chemical formula of sodium …
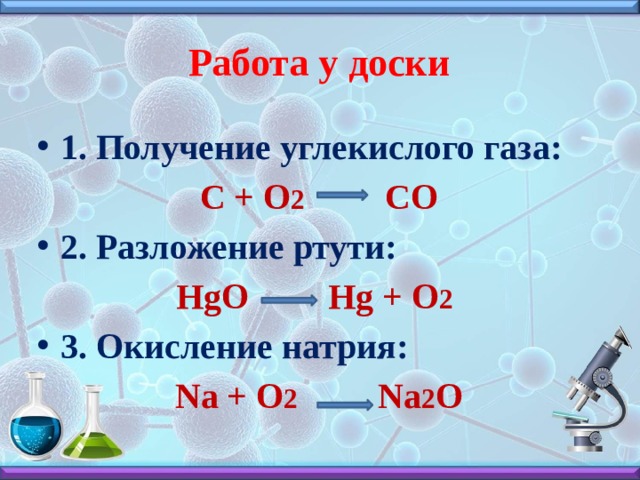 For example, in the reaction of hydrogen (H₂) with oxygen (O₂) to form water (H₂O), the chemical equation is: However, this equation isn't balanced because the number of atoms for each …
For example, in the reaction of hydrogen (H₂) with oxygen (O₂) to form water (H₂O), the chemical equation is: However, this equation isn't balanced because the number of atoms for each …
Еще по теме:






Еще по теме: