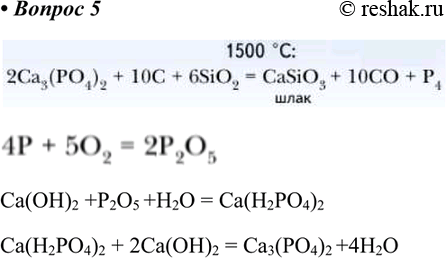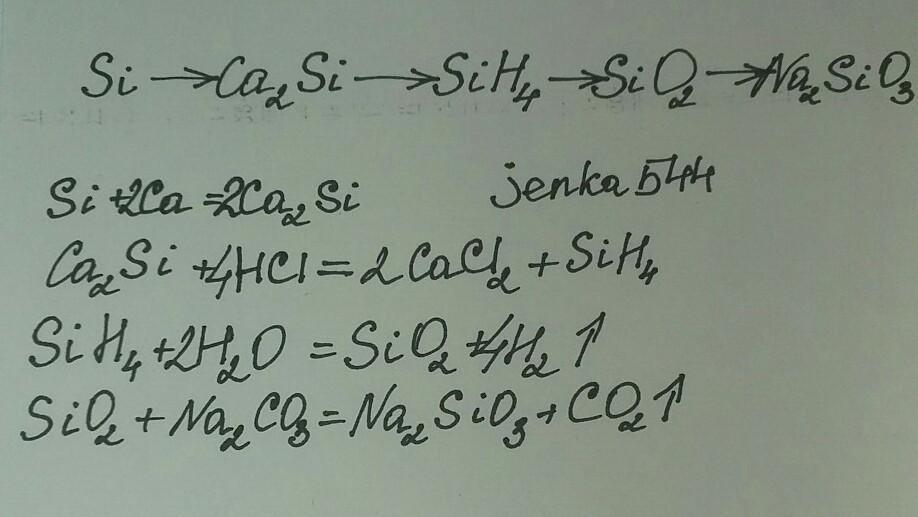Balance the reaction of Ca3(PO4)2 + SiO2 + C = CaSiO3 + P4 + CO using this chemical equation balancer!
Balance the reaction of Ca3(PO4)2 + SiO2 + C = CaSiO3 + P4 + CO using this chemical equation balancer!
 Solved and balanced chemical equation 2 Ca3 (PO4)2 + 10 C + 6 SiO2 → P4 + 6 CaSiO3 + 10 CO with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation 2 Ca3 (PO4)2 + 10 C + 6 SiO2 → P4 + 6 CaSiO3 + 10 CO with completed products. Application for completing products and balancing equations.
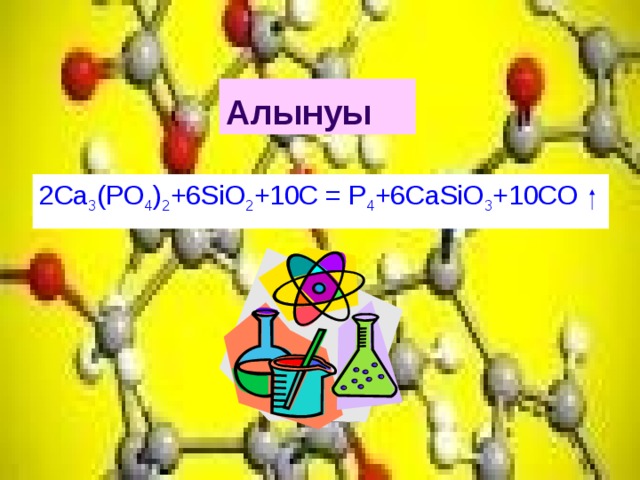
 Вот так 2Ca3(PO4)2 + 6SiO2 + 10C = 6CaSiO3 + P4 +10CO. Ну, или можно перед фосфором поставить коэффициент 4.
Вот так 2Ca3(PO4)2 + 6SiO2 + 10C = 6CaSiO3 + P4 +10CO. Ну, или можно перед фосфором поставить коэффициент 4.
 Ca 3 (PO 4) 2 (Tricalcium Phosphate) Summary of Redox Reaction. 2Ca3(PO4)2 + 6SiO2 + 10C = 6CaSiO3 + P4 + 10CO is a redox reaction where C is oxidized and P is reduced. C is a …
Ca 3 (PO 4) 2 (Tricalcium Phosphate) Summary of Redox Reaction. 2Ca3(PO4)2 + 6SiO2 + 10C = 6CaSiO3 + P4 + 10CO is a redox reaction where C is oxidized and P is reduced. C is a …
 Answer: 2Ca₃ (PO₄)₂ + 10C + 6SiO₂ → 6CaSiO₃ + P₄ + 10CO. Explanation: To balance a chemical reaction, we should apply the law of conservation of mass. Law of …
Answer: 2Ca₃ (PO₄)₂ + 10C + 6SiO₂ → 6CaSiO₃ + P₄ + 10CO. Explanation: To balance a chemical reaction, we should apply the law of conservation of mass. Law of …
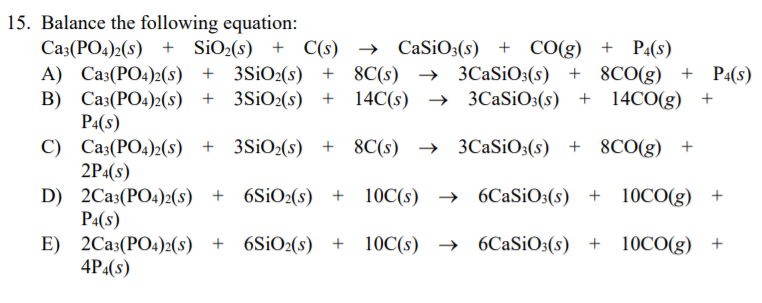
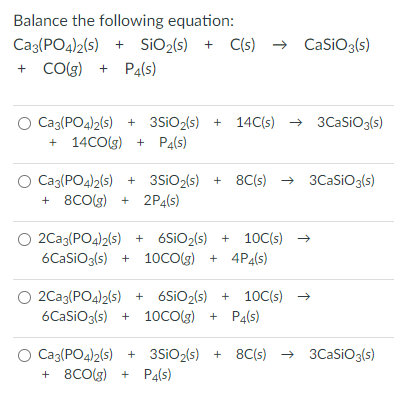 Elemental phosphorus can be prepared from calcium phosphate via the overall reaction 2Ca3(PO4)2_ 6SiO2+10C=6CaSiO3+P4+10CO. (a) Calculate the minimum mass of …
Elemental phosphorus can be prepared from calcium phosphate via the overall reaction 2Ca3(PO4)2_ 6SiO2+10C=6CaSiO3+P4+10CO. (a) Calculate the minimum mass of …
 Получаем. 2Ca3 (PO4)2 + 12C + 6SiO2 = 6CaSiO3 + 12CO + P4. Похожие вопросы. Пользователь Katrine Zabello задал вопрос в категории Естественные науки и получил на него 2 ответа.
Получаем. 2Ca3 (PO4)2 + 12C + 6SiO2 = 6CaSiO3 + 12CO + P4. Похожие вопросы. Пользователь Katrine Zabello задал вопрос в категории Естественные науки и получил на него 2 ответа.
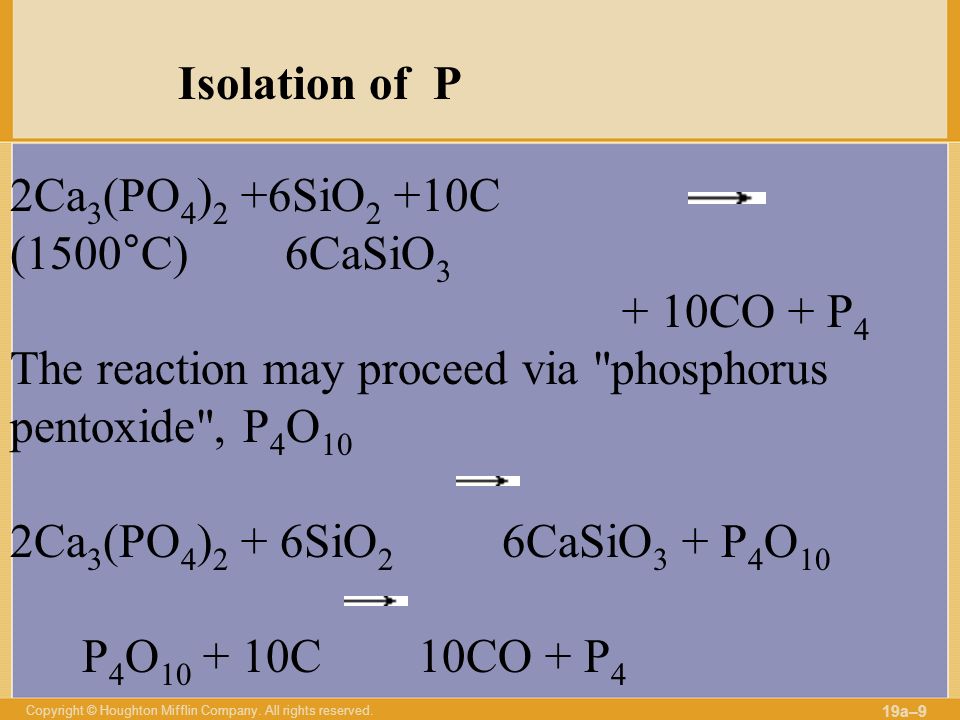
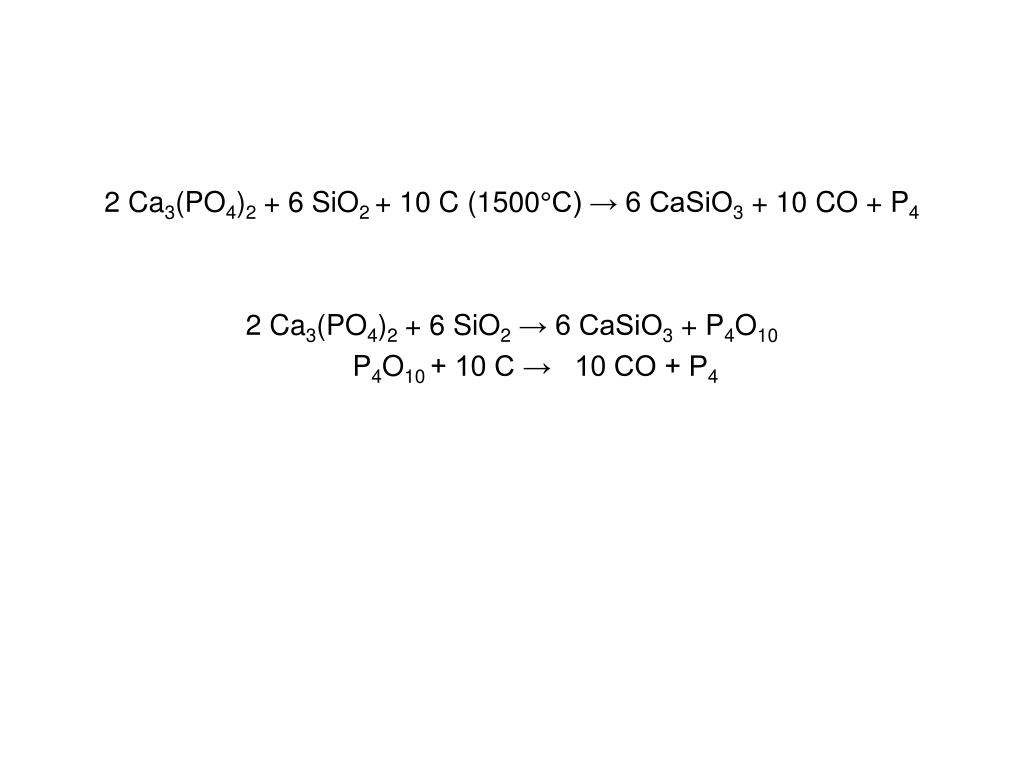 2Ca3(PO4)2 + 10C + 6SiO2 → P4 + 10CO + 6CaSiO3. Реакция взаимодействия фосфата кальция, углерода (в виде кокса) и оксида кремния (IV). В результате реакции …
2Ca3(PO4)2 + 10C + 6SiO2 → P4 + 10CO + 6CaSiO3. Реакция взаимодействия фосфата кальция, углерода (в виде кокса) и оксида кремния (IV). В результате реакции …
 2Ca3(PO4)2 + 6SiO2 + 10C ---> 6CaSiO3 + P4 + 10CO (Электротермический процесс) 4P + 5O2 --->2 P2O5 P2O5+ Ca(OH)2 + H2O -----> Ca(H2PO4)2
2Ca3(PO4)2 + 6SiO2 + 10C ---> 6CaSiO3 + P4 + 10CO (Электротермический процесс) 4P + 5O2 --->2 P2O5 P2O5+ Ca(OH)2 + H2O -----> Ca(H2PO4)2
 The equation for the preparation of phosphorus in an electric furnace is: 2Ca3 (PO4)2 + 6SiO2 + 10C=6CaSiO3 +10CO + P4. Determine the mass of phosphorus which can be obtained from …
The equation for the preparation of phosphorus in an electric furnace is: 2Ca3 (PO4)2 + 6SiO2 + 10C=6CaSiO3 +10CO + P4. Determine the mass of phosphorus which can be obtained from …
 Balanced Chemical Equation. 2 Ca 3 (PO 4) 2 + 10 C + 6 SiO 2 → 6 CaSiO 3 + P 4 + 10 CO. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. …
Balanced Chemical Equation. 2 Ca 3 (PO 4) 2 + 10 C + 6 SiO 2 → 6 CaSiO 3 + P 4 + 10 CO. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. …
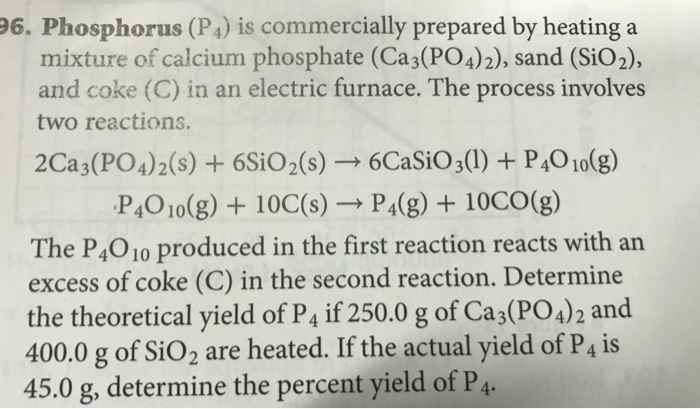
 Phosphorus, P4. can be prepared from calcium phosphate by the reaction 3410 g 1695 g 660 g 2Ca3 (PO4)2 + 6SiO2 10C → 6CaSiO3 + P4 +10CO 310 g/mol 60.1 g/mol 12.0 g/mol The …
Phosphorus, P4. can be prepared from calcium phosphate by the reaction 3410 g 1695 g 660 g 2Ca3 (PO4)2 + 6SiO2 10C → 6CaSiO3 + P4 +10CO 310 g/mol 60.1 g/mol 12.0 g/mol The …
 Calculate mass (in grams) of Ca3(PO4)2 and of KCl that can be produced by mixing 5g of CaCl2 with 8g of K3PO4 according to 3CaCl2 + 2 K3PO4 → Ca3(PO4)2 + 6KCI (M.Wt CaCl2=110, …
Calculate mass (in grams) of Ca3(PO4)2 and of KCl that can be produced by mixing 5g of CaCl2 with 8g of K3PO4 according to 3CaCl2 + 2 K3PO4 → Ca3(PO4)2 + 6KCI (M.Wt CaCl2=110, …
 2Ca3(PO4)2 (s) + 10C (s) + 6SiO2 (s) -> 6CaSiO3 (s) + 10CO (g) + P4 (g) explain the role of each of the reagents in this reaction including why it is used instead of other possible reagents. …
2Ca3(PO4)2 (s) + 10C (s) + 6SiO2 (s) -> 6CaSiO3 (s) + 10CO (g) + P4 (g) explain the role of each of the reagents in this reaction including why it is used instead of other possible reagents. …
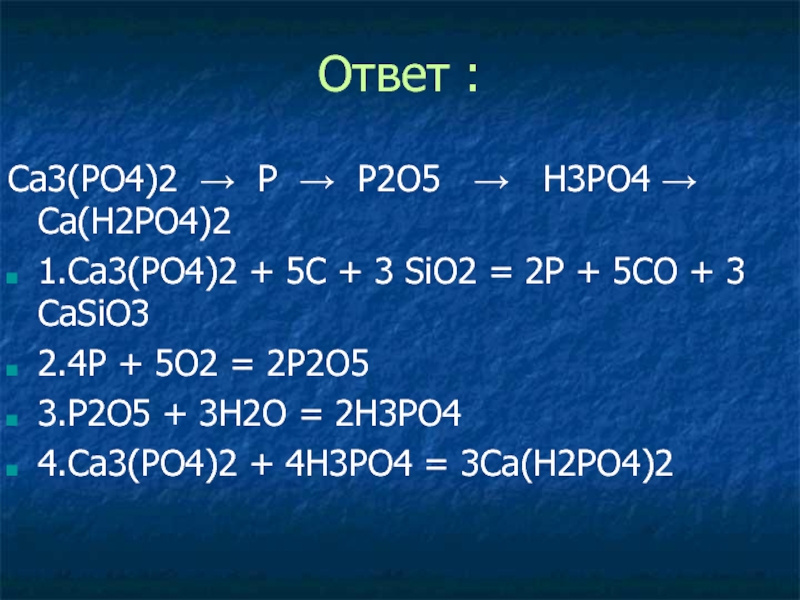
 ca3(po4)2→p→ca3p2→ph3→p2o5 →h3po4→ca3(po4)2→ h3po4 → na2hpo4 1. 2ca3(po4)2 + 10c(кокс) + 6sio2 = 6casio3 + p4 + 10co 2. 3ca + 2p(красн. ) = ca3p2 3. ca3p2 + 6h2o = …
ca3(po4)2→p→ca3p2→ph3→p2o5 →h3po4→ca3(po4)2→ h3po4 → na2hpo4 1. 2ca3(po4)2 + 10c(кокс) + 6sio2 = 6casio3 + p4 + 10co 2. 3ca + 2p(красн. ) = ca3p2 3. ca3p2 + 6h2o = …
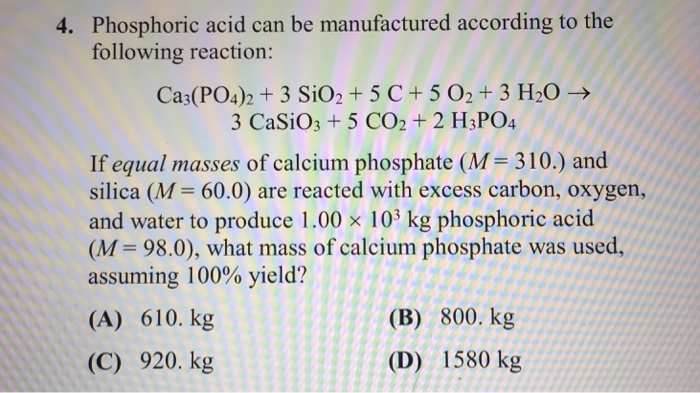 Phosphorus can be prepared from calcium phosphate by the following reaction: 2Ca3 (PO4)2 (s) + 6SiO2 (s) +10C (s) + 6CaSiO3 (s) + P4 (s) + 10CO (g) Phosphorite is a mineral that contains …
Phosphorus can be prepared from calcium phosphate by the following reaction: 2Ca3 (PO4)2 (s) + 6SiO2 (s) +10C (s) + 6CaSiO3 (s) + P4 (s) + 10CO (g) Phosphorite is a mineral that contains …
Еще по теме:






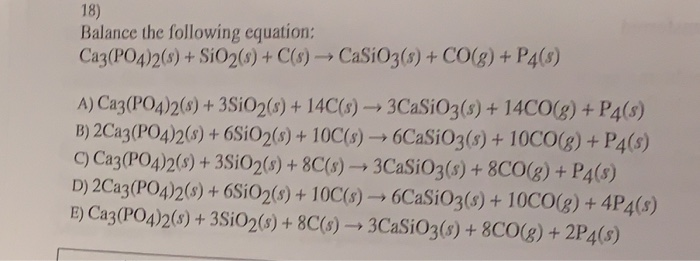






Еще по теме: