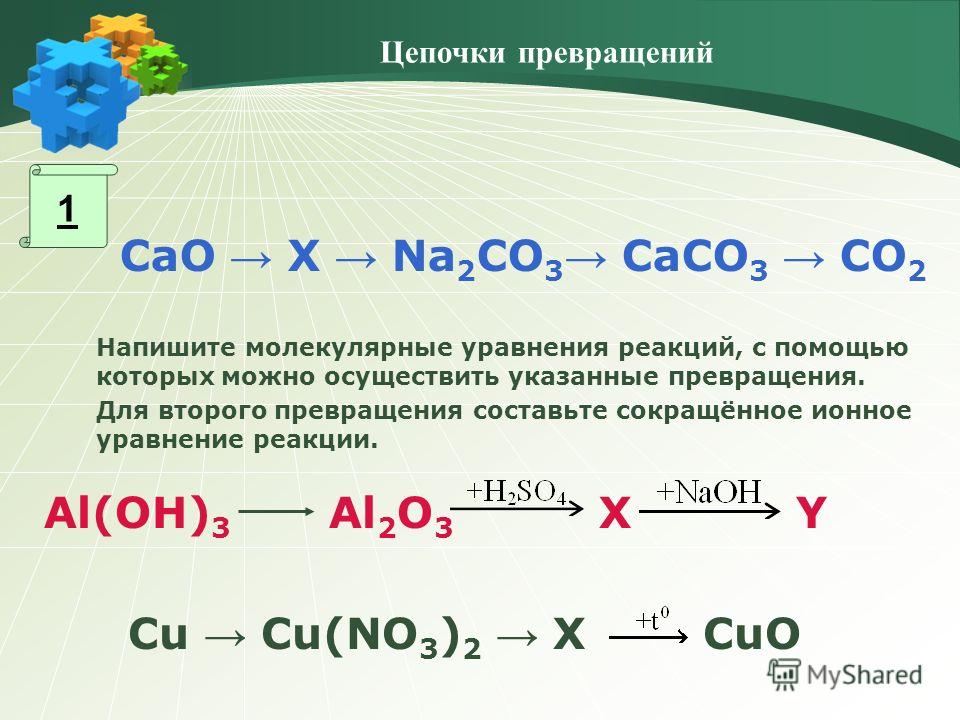
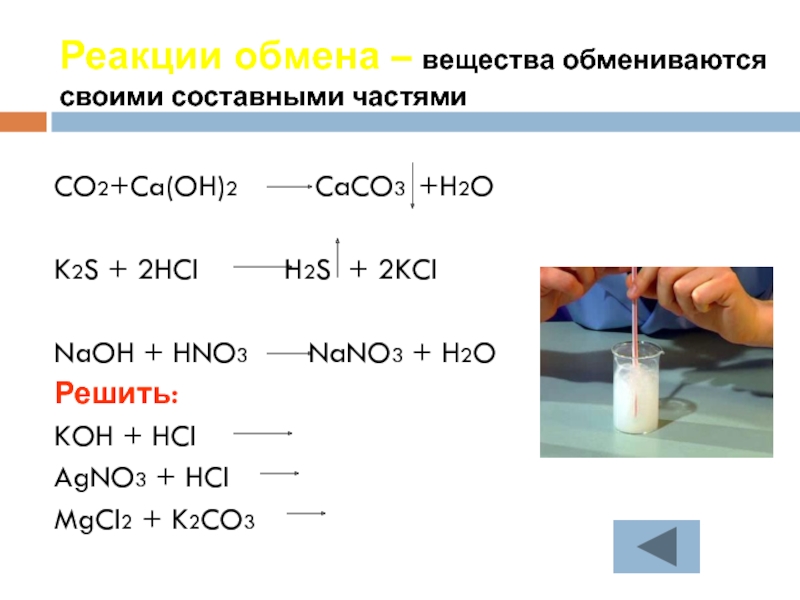
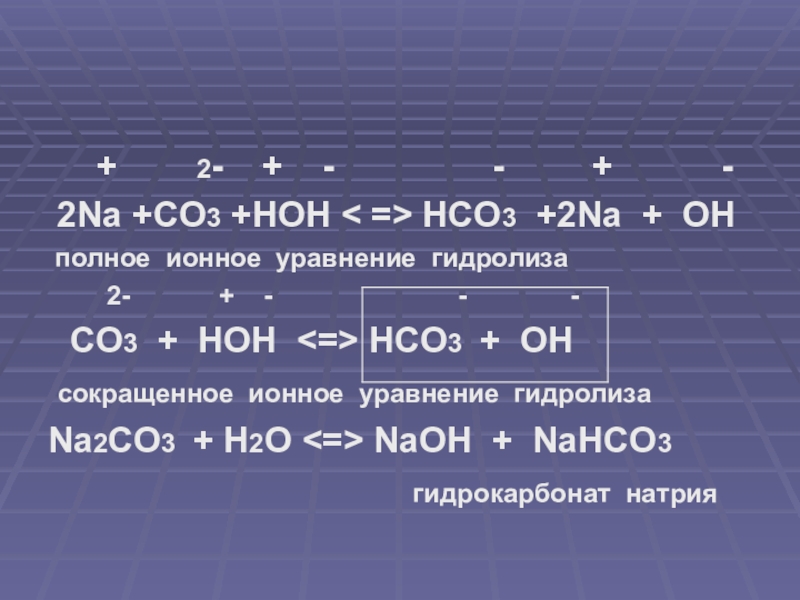
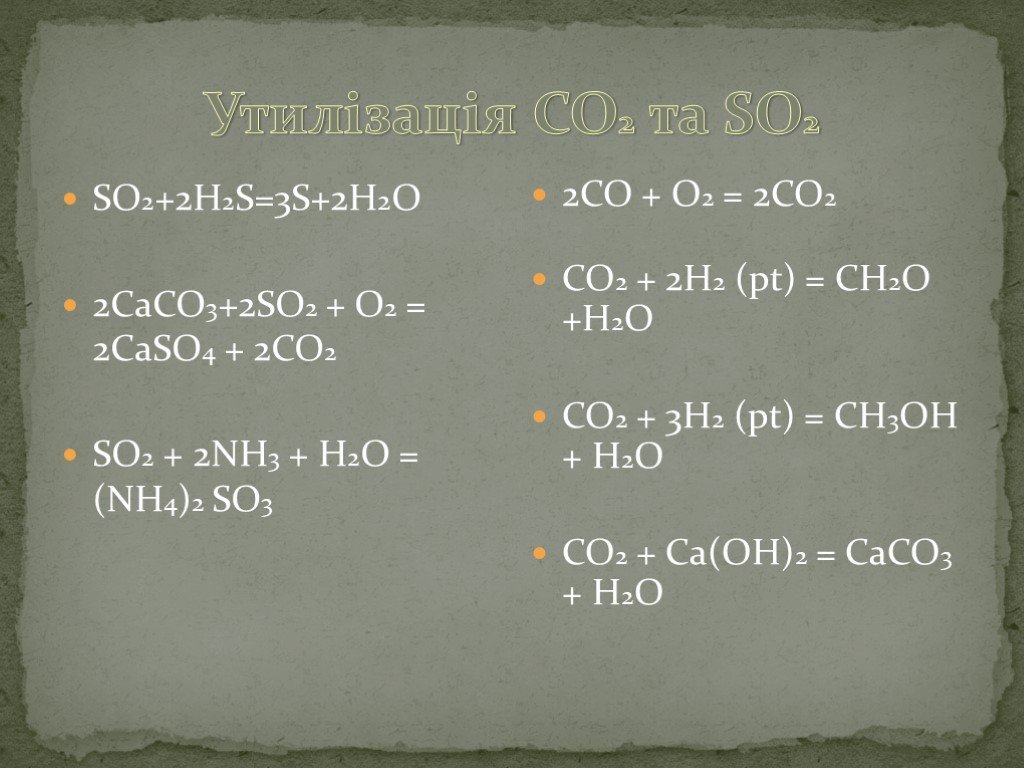
Balance the reaction of CaCO3 + SO2 + O2 = CaSO4 + CO2 using this chemical equation balancer!
Balance the reaction of CaCO3 + SO2 + O2 = CaSO4 + CO2 using this chemical equation balancer!
 Решенное и коэффициентами уравнение реакции 2 SO2 + 2 CaCO3 + O2 → 2 CaSO4 + 2 CO2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов …
Решенное и коэффициентами уравнение реакции 2 SO2 + 2 CaCO3 + O2 → 2 CaSO4 + 2 CO2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов …
 1 CaCO 3 (s) + 1 SO 2 (g) + 1 O 2 (g) = 1 CaSO 4 (s) + 1 CO 2 (g) For each element, we check if the number of atoms is balanced on both sides of the equation. Ca is balanced: 1 atom in …
1 CaCO 3 (s) + 1 SO 2 (g) + 1 O 2 (g) = 1 CaSO 4 (s) + 1 CO 2 (g) For each element, we check if the number of atoms is balanced on both sides of the equation. Ca is balanced: 1 atom in …
 Solved and balanced chemical equation 2 SO2 + 2 CaCO3 + O2 → 2 CaSO4 + 2 CO2 with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation 2 SO2 + 2 CaCO3 + O2 → 2 CaSO4 + 2 CO2 with completed products. Application for completing products and balancing equations.
 To be balanced, every element in SO2 + CaCO3 + O2 = CaSO4 + CO2 must have the same number of atoms on each side of the equation. When using the inspection method (also known …
To be balanced, every element in SO2 + CaCO3 + O2 = CaSO4 + CO2 must have the same number of atoms on each side of the equation. When using the inspection method (also known …
 For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but PhC2H5 + O2 = PhOH + CO2 + H2O will Compound states [like (s) (aq) or (g)] are not required.
For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but PhC2H5 + O2 = PhOH + CO2 + H2O will Compound states [like (s) (aq) or (g)] are not required.
 Решенное и коэффициентами уравнение реакции o2 + 2 so2 + 2 caco3 → 2 co2 + 2 caso4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции o2 + 2 so2 + 2 caco3 → 2 co2 + 2 caso4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Balance the reaction of SO2 + CaCO3 + O2 + H2O = CaSO4*2H2O + CO2 using this chemical equation balancer!
Balance the reaction of SO2 + CaCO3 + O2 + H2O = CaSO4*2H2O + CO2 using this chemical equation balancer!
 Balance equation CaCO3 + SO2 + O2 = CaSO4 + CO2 Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.
Balance equation CaCO3 + SO2 + O2 = CaSO4 + CO2 Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.
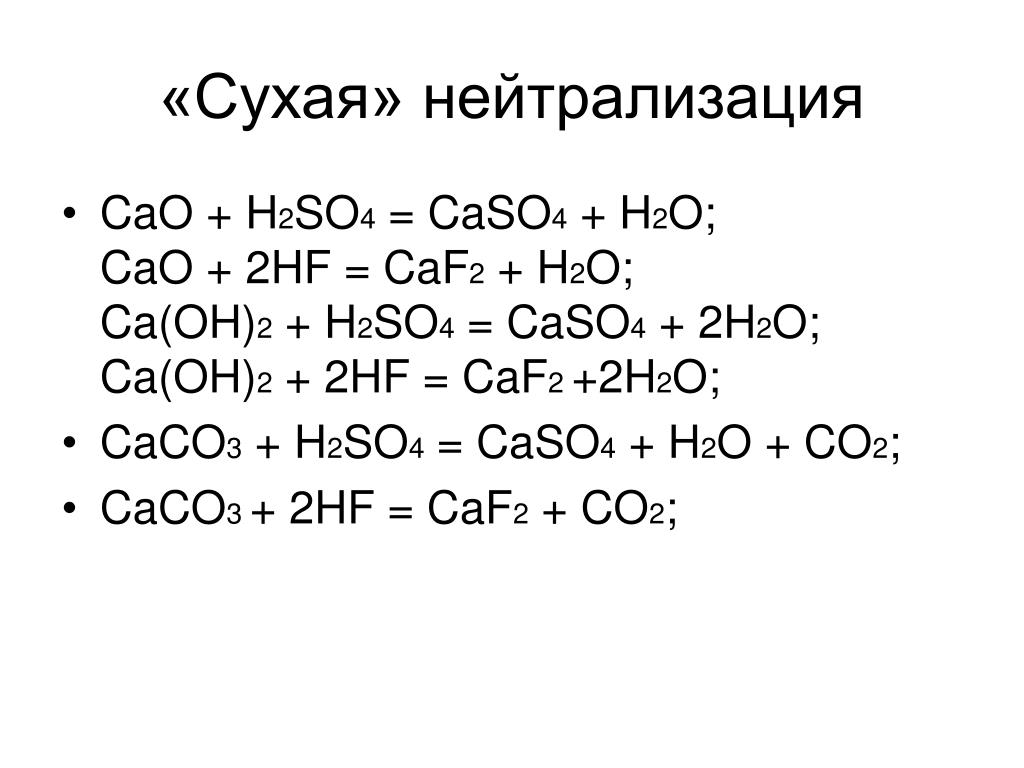
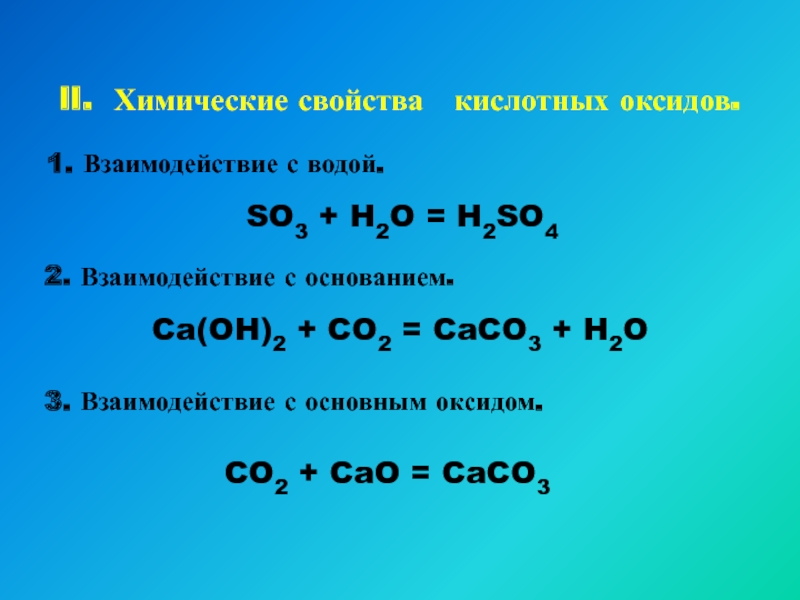
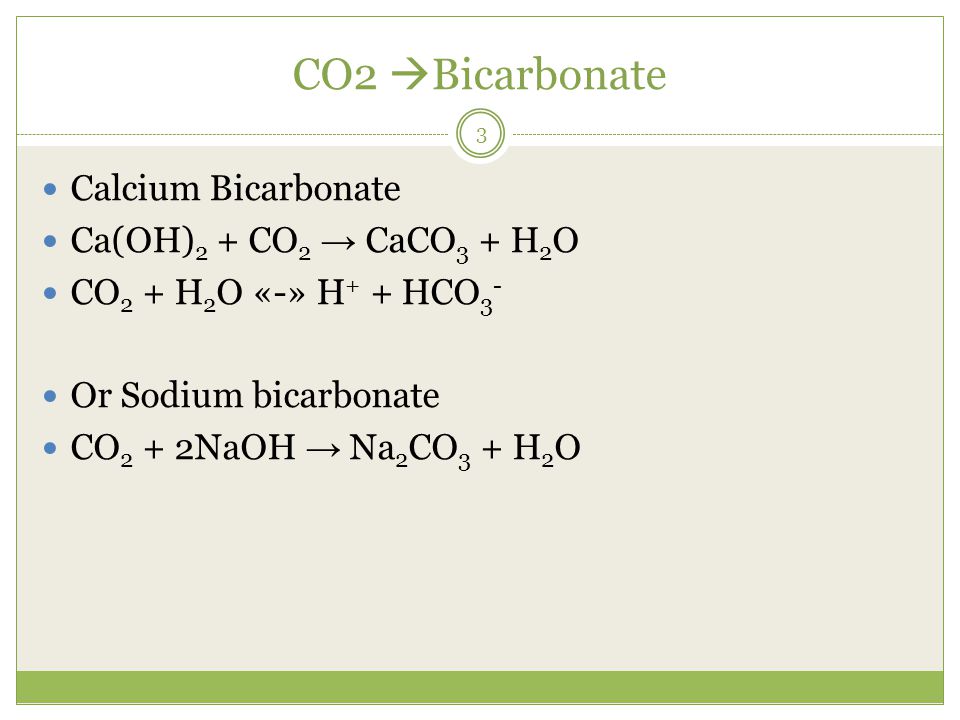
 Calcium oxide and sulfur dioxide may react to give calcium sulfite: $$\ce{CaO + SO2 -> CaSO3}$$ On the other hand alkali metal oxides and alkaline earth metal oxides do …
Calcium oxide and sulfur dioxide may react to give calcium sulfite: $$\ce{CaO + SO2 -> CaSO3}$$ On the other hand alkali metal oxides and alkaline earth metal oxides do …
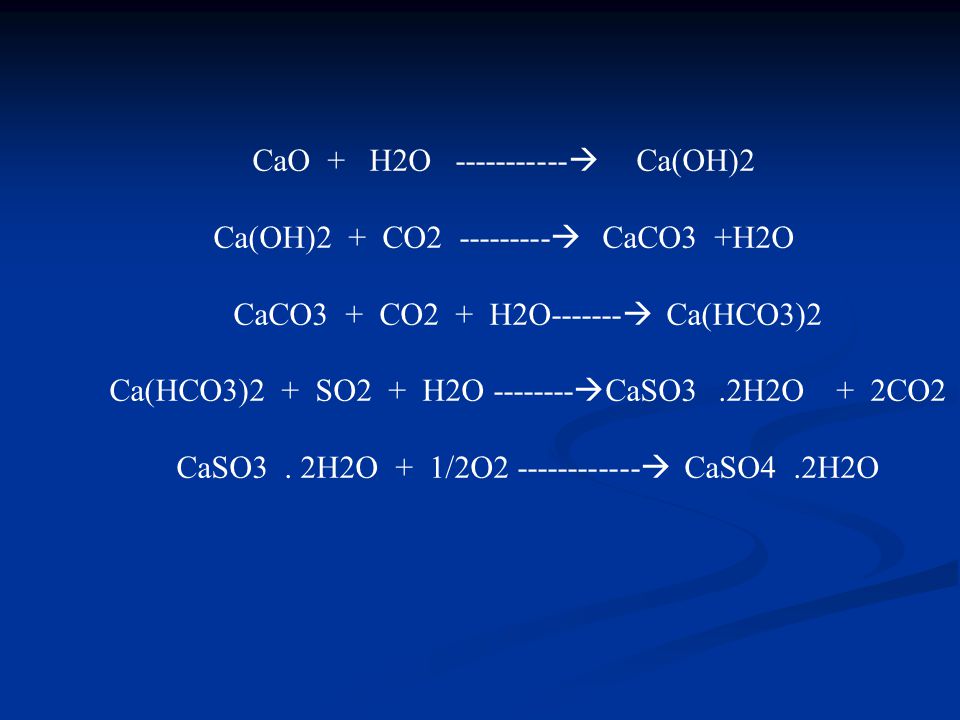
 The reaction of CaCO 3 with SO 2 alone or with O 2 has ben investigated, taking into account previously obtained results on the reaction of CaSO 3 with SO 2 or O 2. The main kinetic …
The reaction of CaCO 3 with SO 2 alone or with O 2 has ben investigated, taking into account previously obtained results on the reaction of CaSO 3 with SO 2 or O 2. The main kinetic …
 Construct the rate of reaction expression for: O_2 + SO_2 + CaCO_3 CO_2 + CaSO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate …
Construct the rate of reaction expression for: O_2 + SO_2 + CaCO_3 CO_2 + CaSO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate …
 Calcium carbonate reacts with sulfur dioxide and oxygen gases to produce calcium sulfate and carbon dioxide. Calculate the number of tons of CaCO3 needed to react completely with 2.20 …
Calcium carbonate reacts with sulfur dioxide and oxygen gases to produce calcium sulfate and carbon dioxide. Calculate the number of tons of CaCO3 needed to react completely with 2.20 …
 In this study, we investigated the multiphase reaction of SO2 with an O2 ∕ NO2 mixture on single CaCO3 particles using Micro-Raman spectroscopy. The reaction converted the CaCO3 …
In this study, we investigated the multiphase reaction of SO2 with an O2 ∕ NO2 mixture on single CaCO3 particles using Micro-Raman spectroscopy. The reaction converted the CaCO3 …
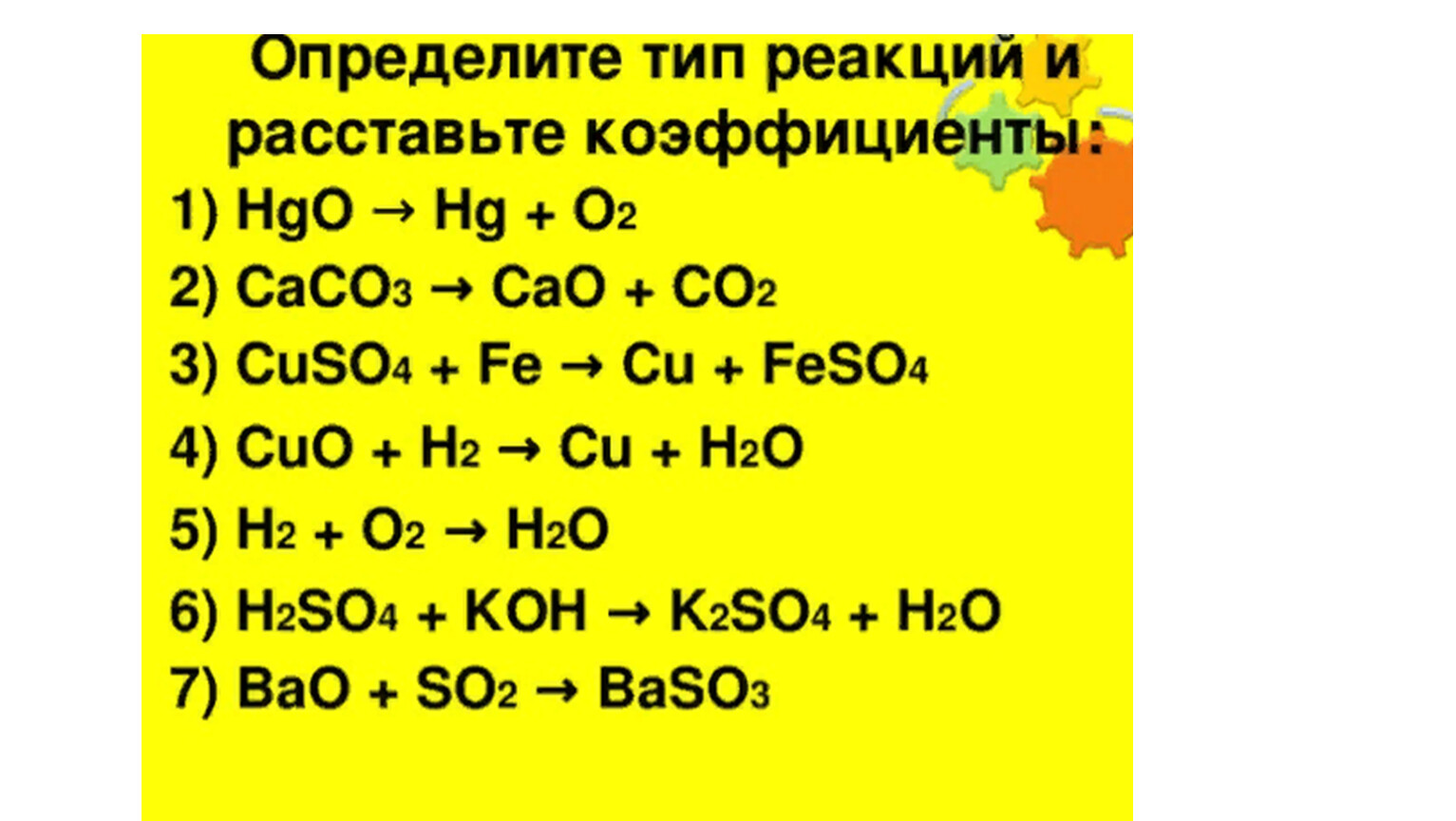
 Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
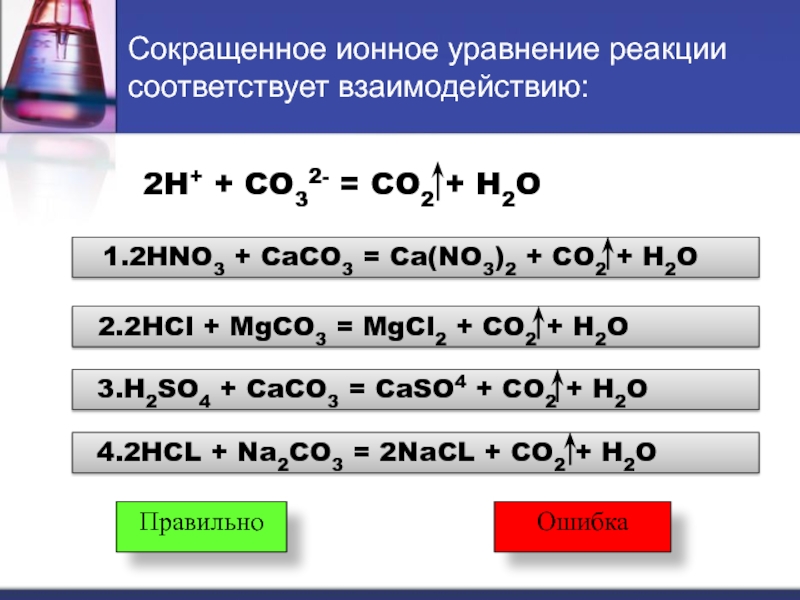
 Oxygen + 2 Calcium carbonate + 2 Sulfur dioxide = 2 Gypsum + 2 Carbon dioxide Full ionic equation O 2 + 2 CaCO 3 + 2 SO 2 = 2 Ca { +2 } + 2 SO 4 { -2 } + 2 CO 2
Oxygen + 2 Calcium carbonate + 2 Sulfur dioxide = 2 Gypsum + 2 Carbon dioxide Full ionic equation O 2 + 2 CaCO 3 + 2 SO 2 = 2 Ca { +2 } + 2 SO 4 { -2 } + 2 CO 2
Еще по теме:
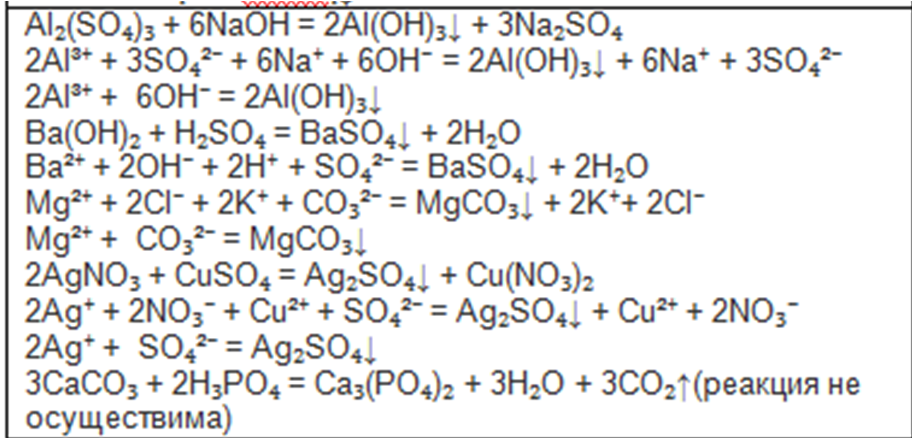






Еще по теме: