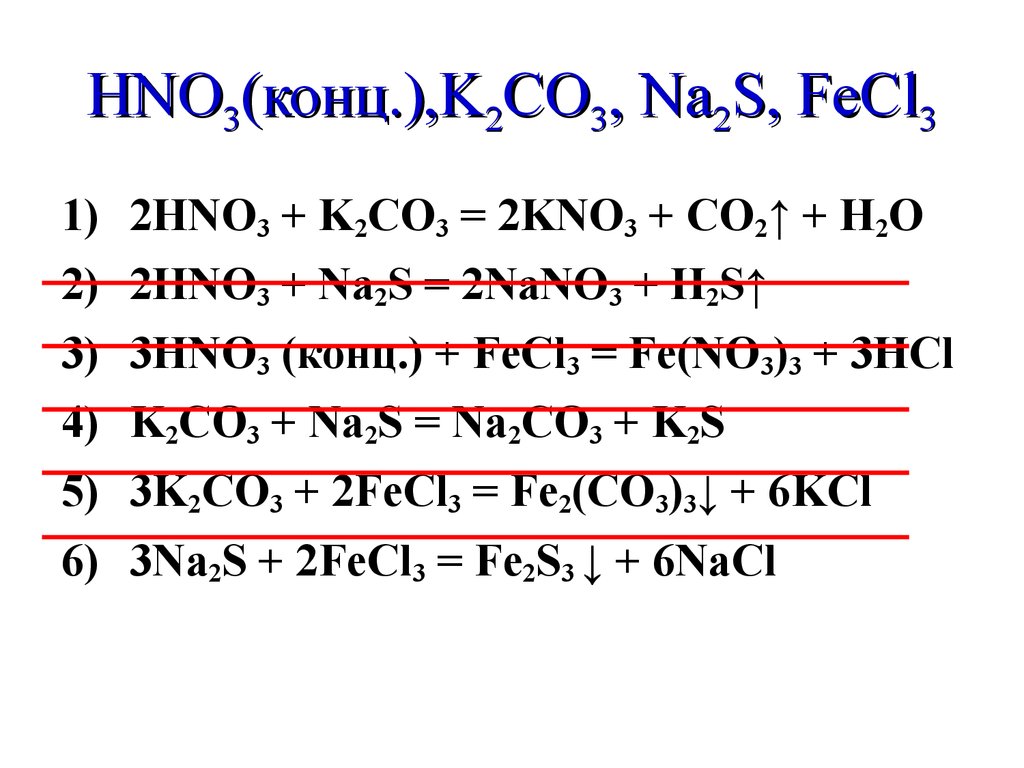
 2 Fe Cl 3 + 3 Na 2 S → 2 Fe S + S + 6 Na Cl. Это окислительно-восстановительная (редокс) реакция: Na2S является восстановителем, FeCl3 является окислителем. Решенное и …
2 Fe Cl 3 + 3 Na 2 S → 2 Fe S + S + 6 Na Cl. Это окислительно-восстановительная (редокс) реакция: Na2S является восстановителем, FeCl3 является окислителем. Решенное и …
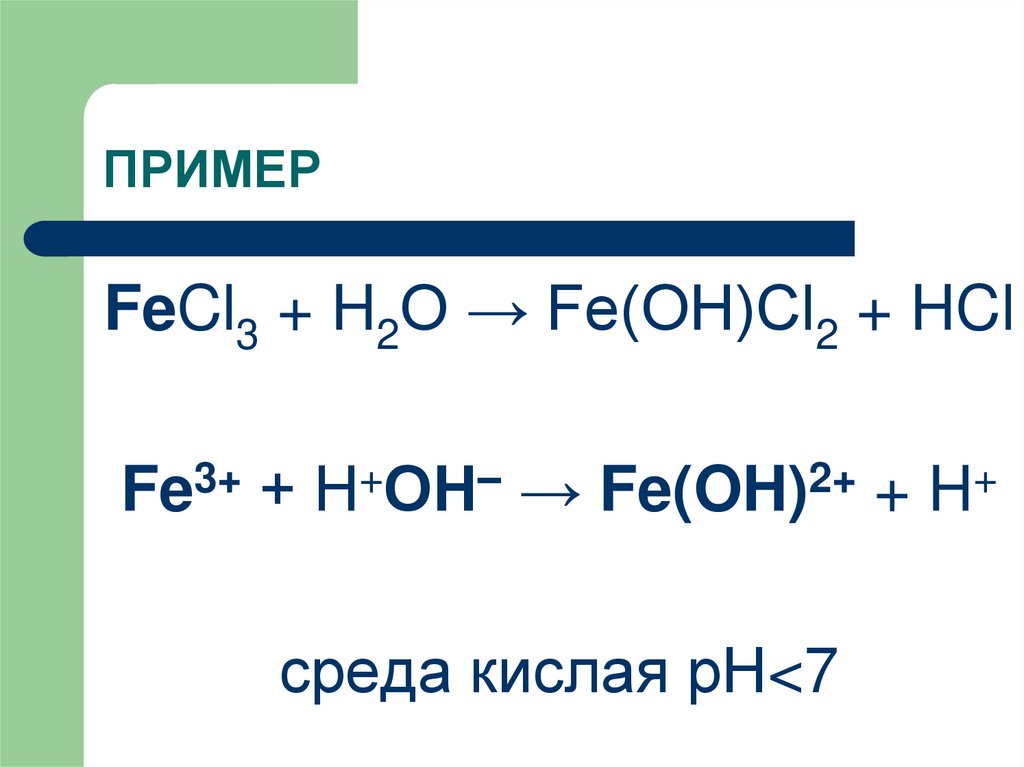
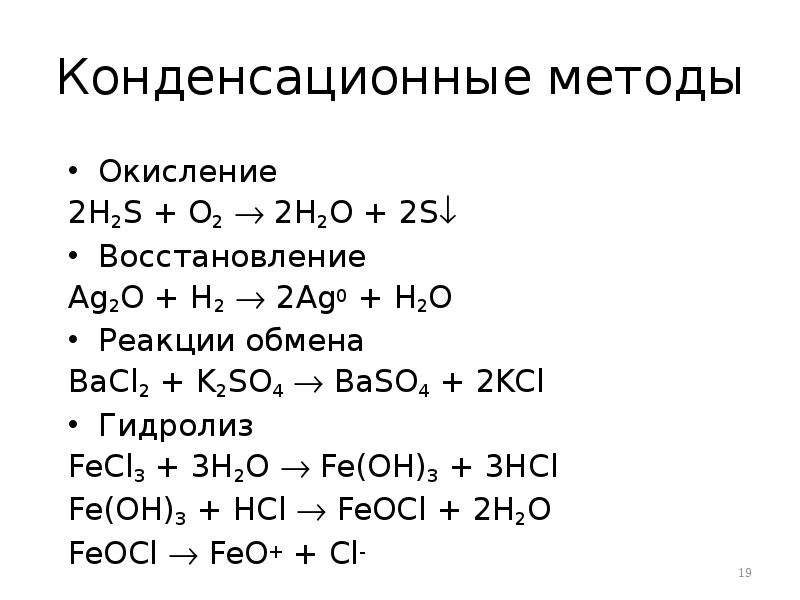
 Реакции хлорида железа (III): гидролиз, ионный обмен, обмен с гидролизом, обмен с ОВР, замещение металла, восстановление соли, конпропорционирование, восстановление …
Реакции хлорида железа (III): гидролиз, ионный обмен, обмен с гидролизом, обмен с ОВР, замещение металла, восстановление соли, конпропорционирование, восстановление …
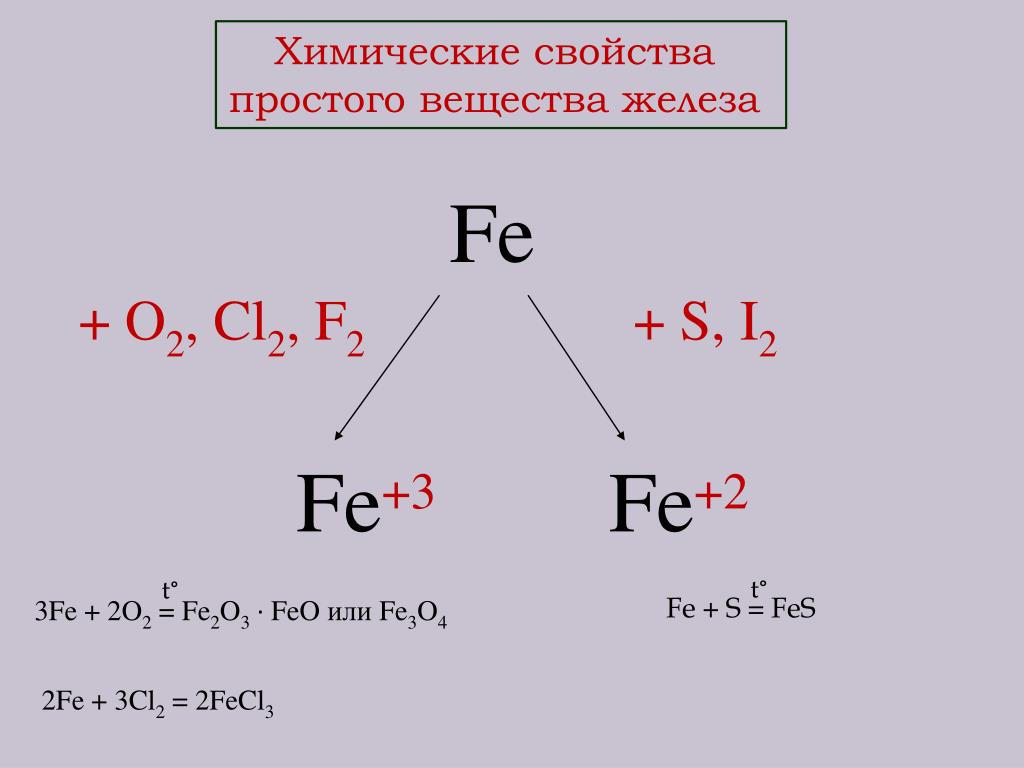
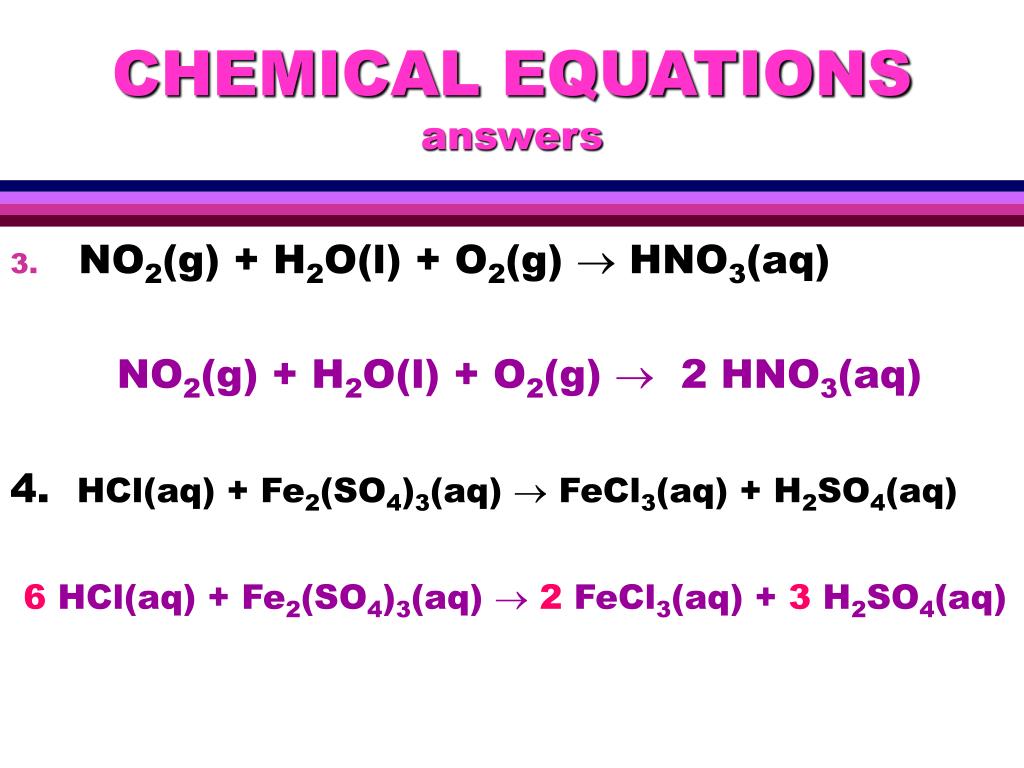
 1 Fe(s) + 1 Cl 2 (g) = 1 FeCl 3 (s) For each element, we check if the number of atoms is balanced on both sides of the equation. Fe is balanced: 1 atom in reagents and 1 atom in products.
1 Fe(s) + 1 Cl 2 (g) = 1 FeCl 3 (s) For each element, we check if the number of atoms is balanced on both sides of the equation. Fe is balanced: 1 atom in reagents and 1 atom in products.

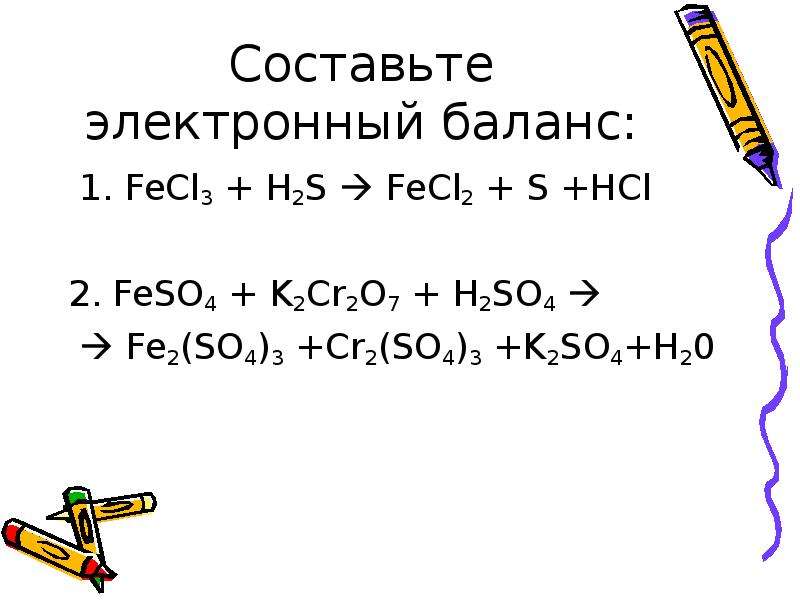
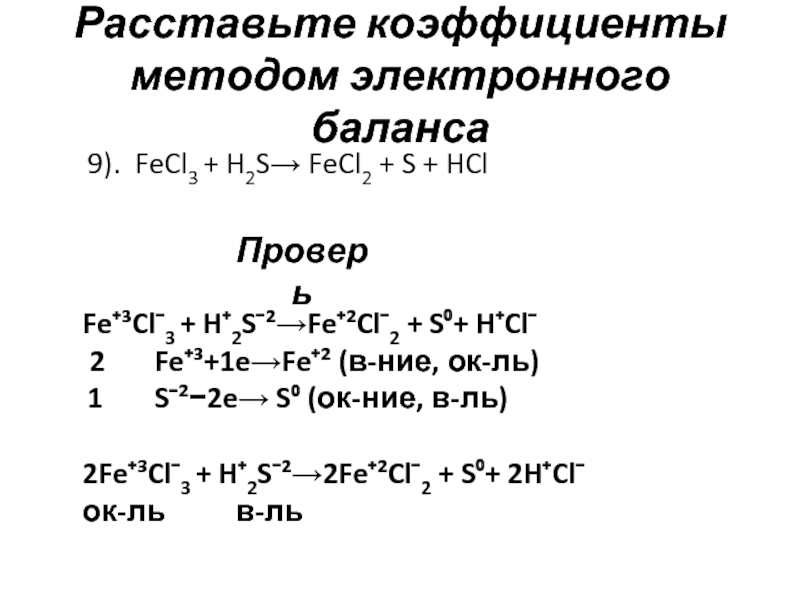 To be balanced, every element in FeCl3 + H2S = FeCl2 + HCl + S must have the same number of atoms on each side of the equation. When using the inspection method (also known as the …
To be balanced, every element in FeCl3 + H2S = FeCl2 + HCl + S must have the same number of atoms on each side of the equation. When using the inspection method (also known as the …
 Ученик. Сумма коэффициентов в уравнении реакции Fe + Cl2 → FeCl3 равна. Нравится. Показать еще 1 ответ. Пользователь Lizay задал вопрос в категории Другие предметы и …
Ученик. Сумма коэффициентов в уравнении реакции Fe + Cl2 → FeCl3 равна. Нравится. Показать еще 1 ответ. Пользователь Lizay задал вопрос в категории Другие предметы и …
 Ferric Chloride | FeCl3 or Cl3Fe | CID 24380 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Ferric Chloride | FeCl3 or Cl3Fe | CID 24380 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
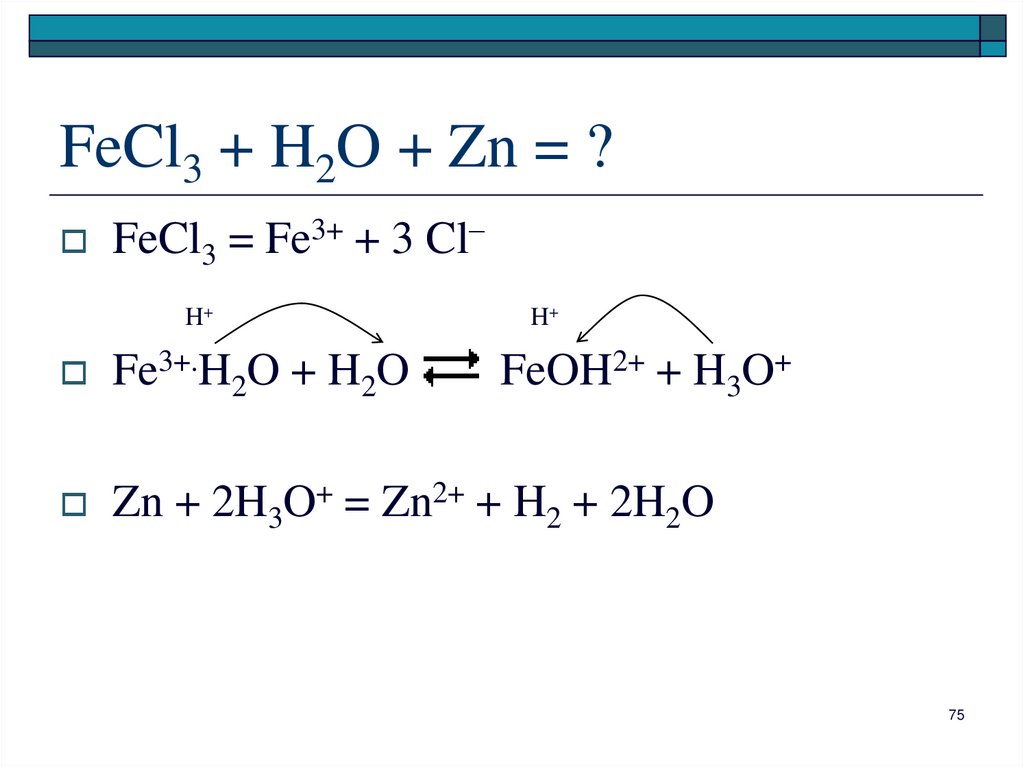
 Aqueous iron (III) chloride serves as a one-electron oxidant illustrated by its reaction with copper (I) chloride to give copper (II) chloride and iron (II) chloride. FeCl3 + CuCl → FeCl2 + CuCl2. This fundamental reaction is relevant to the …
Aqueous iron (III) chloride serves as a one-electron oxidant illustrated by its reaction with copper (I) chloride to give copper (II) chloride and iron (II) chloride. FeCl3 + CuCl → FeCl2 + CuCl2. This fundamental reaction is relevant to the …
 1 FeCl 3 + 1 S = 1 FeCl 3 S Для каждого элемента мы проверяем, сбалансировано ли количество атомов в обеих частях уравнения. Fe сбалансирован: 1 атом в реагентах и 1 …
1 FeCl 3 + 1 S = 1 FeCl 3 S Для каждого элемента мы проверяем, сбалансировано ли количество атомов в обеих частях уравнения. Fe сбалансирован: 1 атом в реагентах и 1 …
 Solved and balanced chemical equation 2 FeCl3 + H2S → 2 FeCl2 + S + 2 HCl with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation 2 FeCl3 + H2S → 2 FeCl2 + S + 2 HCl with completed products. Application for completing products and balancing equations.
 Решенное и коэффициентами уравнение реакции 2 FeCl3 + H2S → 2 FeCl2 + S + 2 HCl с дополненными продуктами.
Решенное и коэффициентами уравнение реакции 2 FeCl3 + H2S → 2 FeCl2 + S + 2 HCl с дополненными продуктами.
 Ferric chloride is a dark colour crystal with the oxidation state of iron being +3. It is also called Iron (III) chloride or Molysite. It is an iron coordination entity which functions as an astringent and …
Ferric chloride is a dark colour crystal with the oxidation state of iron being +3. It is also called Iron (III) chloride or Molysite. It is an iron coordination entity which functions as an astringent and …
 Ferric chloride can be prepared by dissolving hematite in hydrochloric acid: FeX2OX3 +6HCl 2FeClX3 +3HX2O (1) (1) F e X 2 O X 3 + 6 H C l 2 F e C l X 3 + 3 H X 2 O. …
Ferric chloride can be prepared by dissolving hematite in hydrochloric acid: FeX2OX3 +6HCl 2FeClX3 +3HX2O (1) (1) F e X 2 O X 3 + 6 H C l 2 F e C l X 3 + 3 H X 2 O. …
 Iron (III) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula FeCl3. It is a moderately strong Lewis acid and many of its applications …
Iron (III) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula FeCl3. It is a moderately strong Lewis acid and many of its applications …
 S is balanced: 3 atoms in reagents and 3 atoms in products. All atoms are now balanced and the whole equation is fully balanced: 2 FeCl 3 (aq) + 3 H 2 S(g) = Fe 2 S 3 (s) + 6 HCl(aq)
S is balanced: 3 atoms in reagents and 3 atoms in products. All atoms are now balanced and the whole equation is fully balanced: 2 FeCl 3 (aq) + 3 H 2 S(g) = Fe 2 S 3 (s) + 6 HCl(aq)
 The leaching of 137Cs and 90Sr radionuclides contaminated soils by solutions of FeCl3 and NH4Cl in batch (static), column (continuous-flow) and field (Chernobyl region) experiments is.
The leaching of 137Cs and 90Sr radionuclides contaminated soils by solutions of FeCl3 and NH4Cl in batch (static), column (continuous-flow) and field (Chernobyl region) experiments is.
Еще по теме:




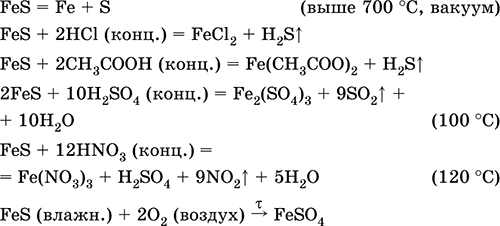

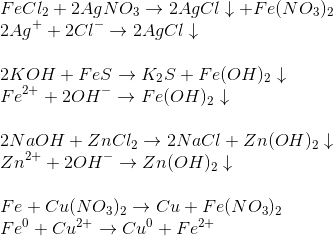

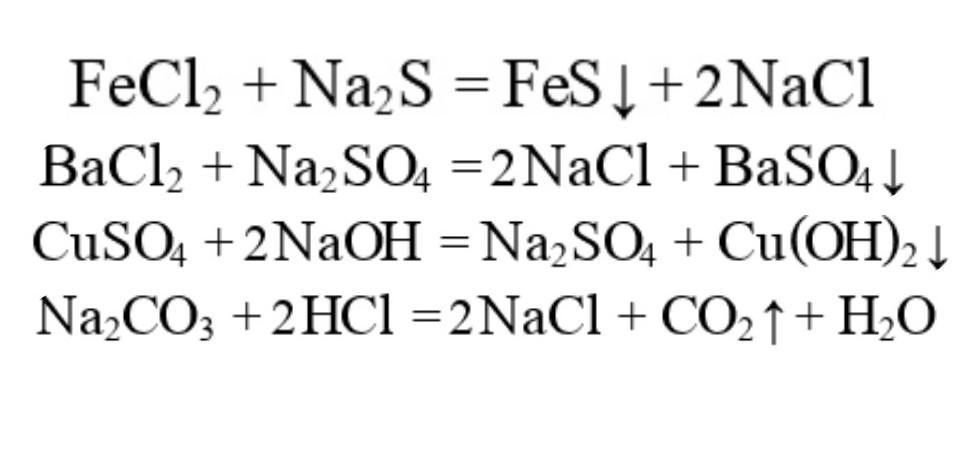



Еще по теме: