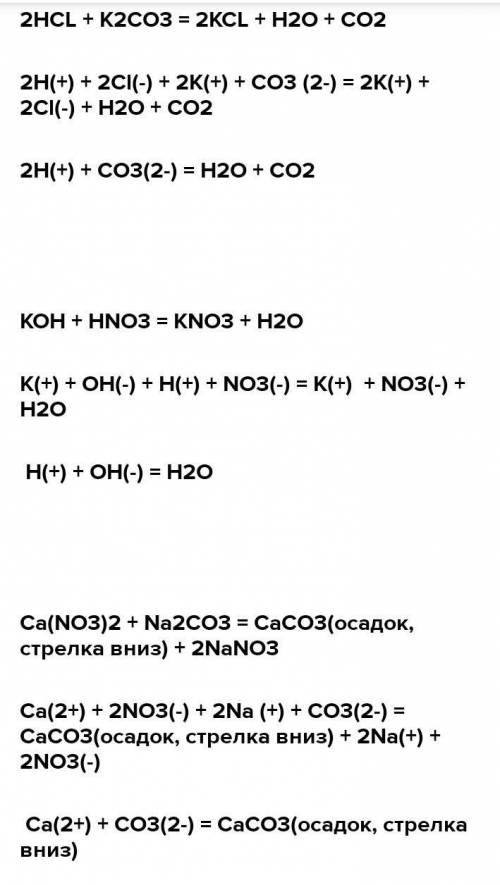
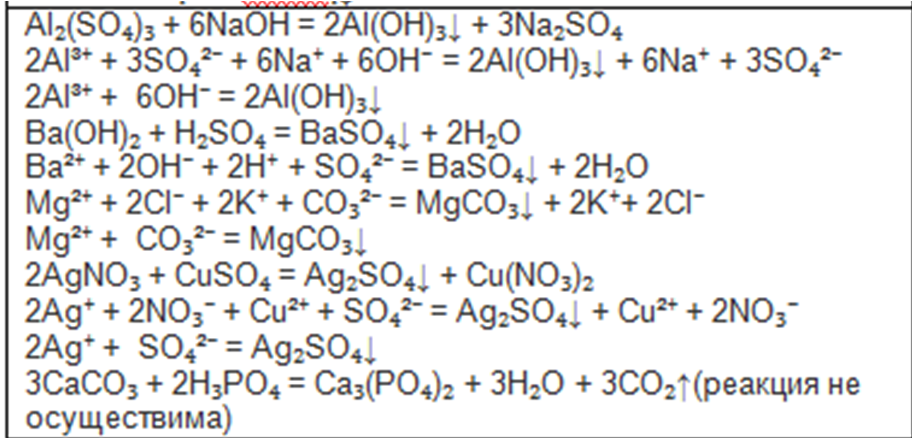Решенное и коэффициентами уравнение реакции k2co3 + ca(no3)2 → 2 kno3 + caco3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции k2co3 + ca(no3)2 → 2 kno3 + caco3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Balance the reaction of Ca(NO3)2 + K2CO3 = CaCO3 + KNO3 using this chemical equation balancer!
Balance the reaction of Ca(NO3)2 + K2CO3 = CaCO3 + KNO3 using this chemical equation balancer!
 1 caco 3 + 1 kno 3 = 1 ca(no 3) 2 + 1 k 2 co 3 For each element, we check if the number of atoms is balanced on both sides of the equation. Ca is balanced: 1 atom in reagents and 1 atom in …
1 caco 3 + 1 kno 3 = 1 ca(no 3) 2 + 1 k 2 co 3 For each element, we check if the number of atoms is balanced on both sides of the equation. Ca is balanced: 1 atom in reagents and 1 atom in …
 В Ca 3 P 2 кальций имеет степень окисления +2, а фосфор имеет степень окисления -3. Определите изменение степени окисления: Кальций переходит от 0 к +2, теряя 2 …
В Ca 3 P 2 кальций имеет степень окисления +2, а фосфор имеет степень окисления -3. Определите изменение степени окисления: Кальций переходит от 0 к +2, теряя 2 …
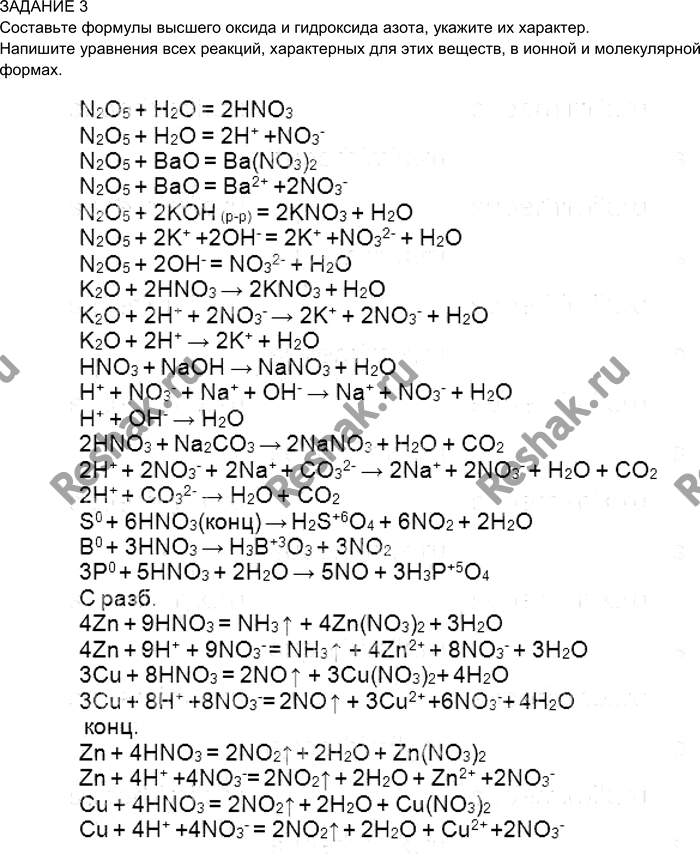
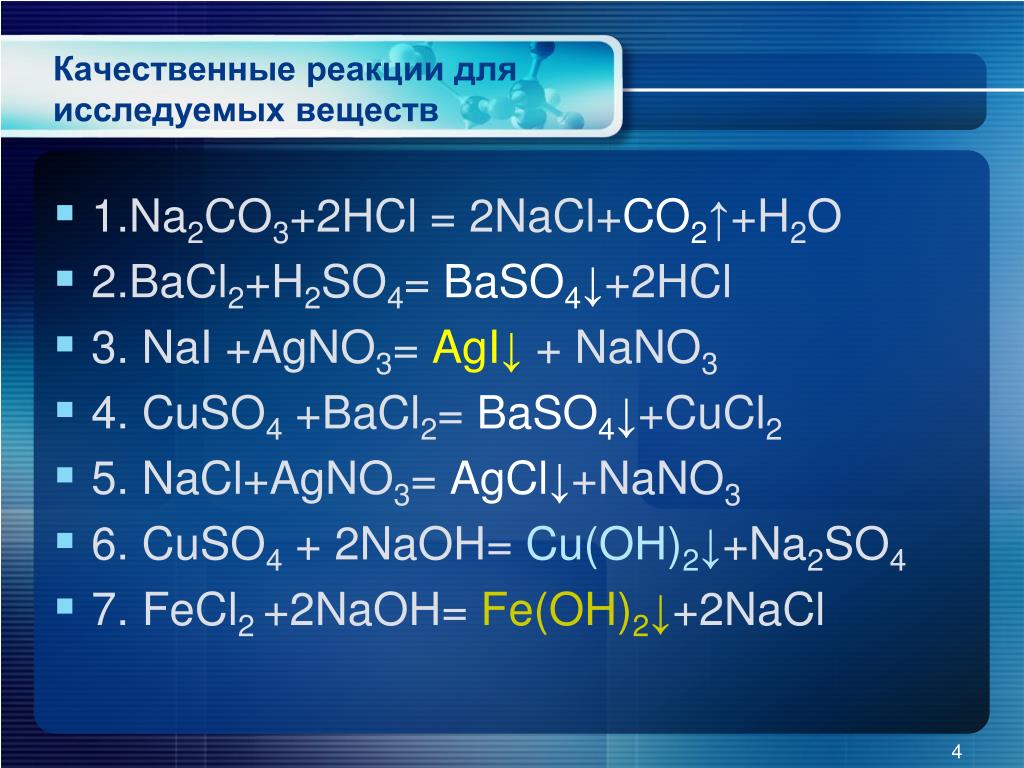
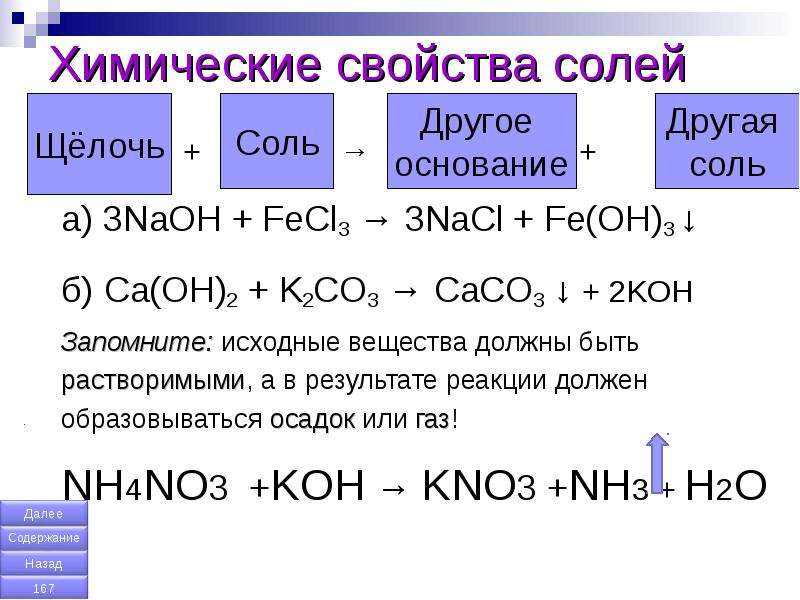
 а) ca(no3)2 + k2co3 →caco3 + kno3, б) ag2so4 + k3po4→ ag3po4 + k2so4, в) hno3 + ba(oh)2→ba(no3)2 + h2o, г) h2so4 + sr(oh)2 →srso4 + h2o
а) ca(no3)2 + k2co3 →caco3 + kno3, б) ag2so4 + k3po4→ ag3po4 + k2so4, в) hno3 + ba(oh)2→ba(no3)2 + h2o, г) h2so4 + sr(oh)2 →srso4 + h2o
 There are three main steps for writing the net ionic equation for Ca (NO3)2 + K2CO3 = CaCO3 + KNO3 (Calcium nitrate + Potassium carbonate). First, we balance the molecular …
There are three main steps for writing the net ionic equation for Ca (NO3)2 + K2CO3 = CaCO3 + KNO3 (Calcium nitrate + Potassium carbonate). First, we balance the molecular …
 CaCO3 + KNO3 = Ca (NO3)2 + K2CO3 is a Double Displacement (Metathesis) reaction where one mole of solid Calcium Carbonate [CaCO 3] and two moles of aqueous Potassium Nitrate …
CaCO3 + KNO3 = Ca (NO3)2 + K2CO3 is a Double Displacement (Metathesis) reaction where one mole of solid Calcium Carbonate [CaCO 3] and two moles of aqueous Potassium Nitrate …
 Construct the equilibrium constant, K, expression for: K_2CO_3 + Ca(NO_3)_2 KNO_3 + CaCO_3 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • …
Construct the equilibrium constant, K, expression for: K_2CO_3 + Ca(NO_3)_2 KNO_3 + CaCO_3 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • …
 Example: Fe{3+} + I{-} = Fe{2+} + I2; Substitute immutable groups in chemical compounds to avoid ambiguity. For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be …
Example: Fe{3+} + I{-} = Fe{2+} + I2; Substitute immutable groups in chemical compounds to avoid ambiguity. For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be …
 1 KNO 3 (aq) + 1 CaCO 3 = 1 Ca(NO 3) 2 (aq) + 1 K 2 CO 3 For each element, we check if the number of atoms is balanced on both sides of the equation. K is not balanced: 1 atom in …
1 KNO 3 (aq) + 1 CaCO 3 = 1 Ca(NO 3) 2 (aq) + 1 K 2 CO 3 For each element, we check if the number of atoms is balanced on both sides of the equation. K is not balanced: 1 atom in …
 Ca (NO3)2 (aq) + K2CO3 (aq) --> CaCO3 (s) + 2 KNO3 (aq) 50 mL of 1.000 M Ca (NO3)2 was reacted with excess potassium carbonate. What mass of calcium carbonate will be made? …
Ca (NO3)2 (aq) + K2CO3 (aq) --> CaCO3 (s) + 2 KNO3 (aq) 50 mL of 1.000 M Ca (NO3)2 was reacted with excess potassium carbonate. What mass of calcium carbonate will be made? …
 1 K 2 CO 3 + 1 Ca(NO 3) 2 *4H 2 O = 1 CaCO 3 + 1 KNO 3 + 1 H 2 O For each element, we check if the number of atoms is balanced on both sides of the equation. K is not balanced: 2 …
1 K 2 CO 3 + 1 Ca(NO 3) 2 *4H 2 O = 1 CaCO 3 + 1 KNO 3 + 1 H 2 O For each element, we check if the number of atoms is balanced on both sides of the equation. K is not balanced: 2 …
 1 ca(no 3) 2 *4h 2 o + 1 k 2 co 3 = 1 kno 3 + 1 caco 3 + 1 h 2 o For each element, we check if the number of atoms is balanced on both sides of the equation. Ca is balanced: 1 atom in reagents …
1 ca(no 3) 2 *4h 2 o + 1 k 2 co 3 = 1 kno 3 + 1 caco 3 + 1 h 2 o For each element, we check if the number of atoms is balanced on both sides of the equation. Ca is balanced: 1 atom in reagents …


Еще по теме:


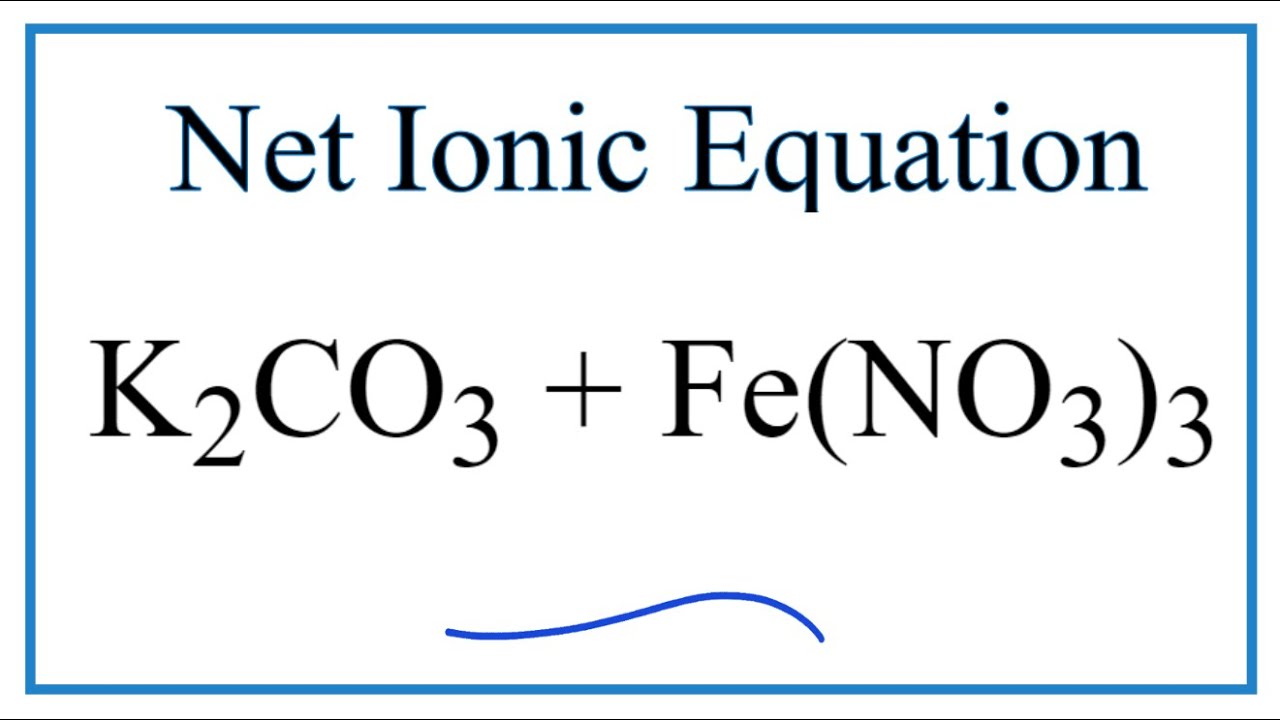

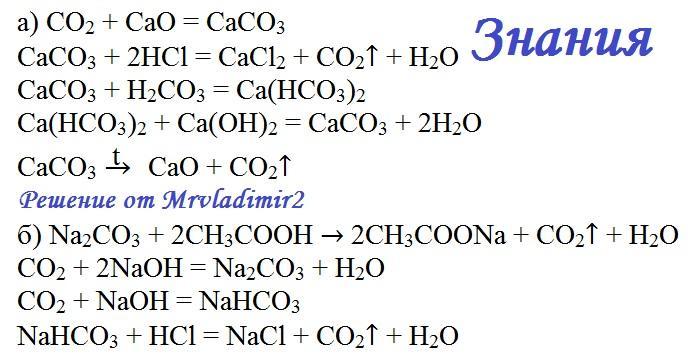







Еще по теме: