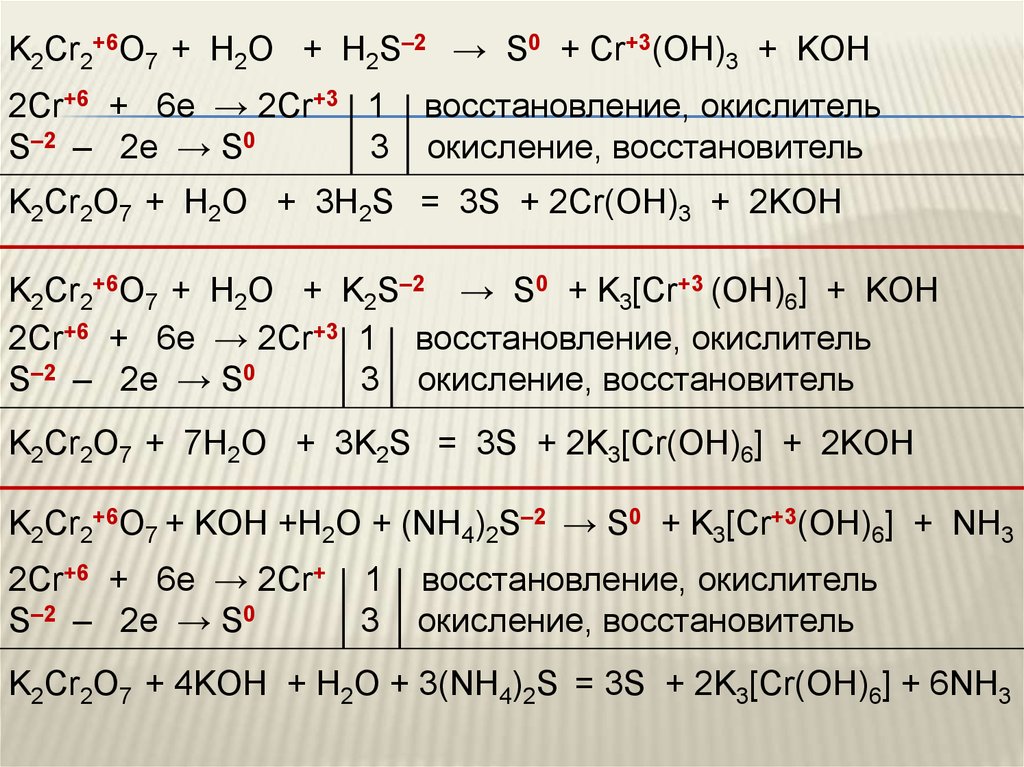
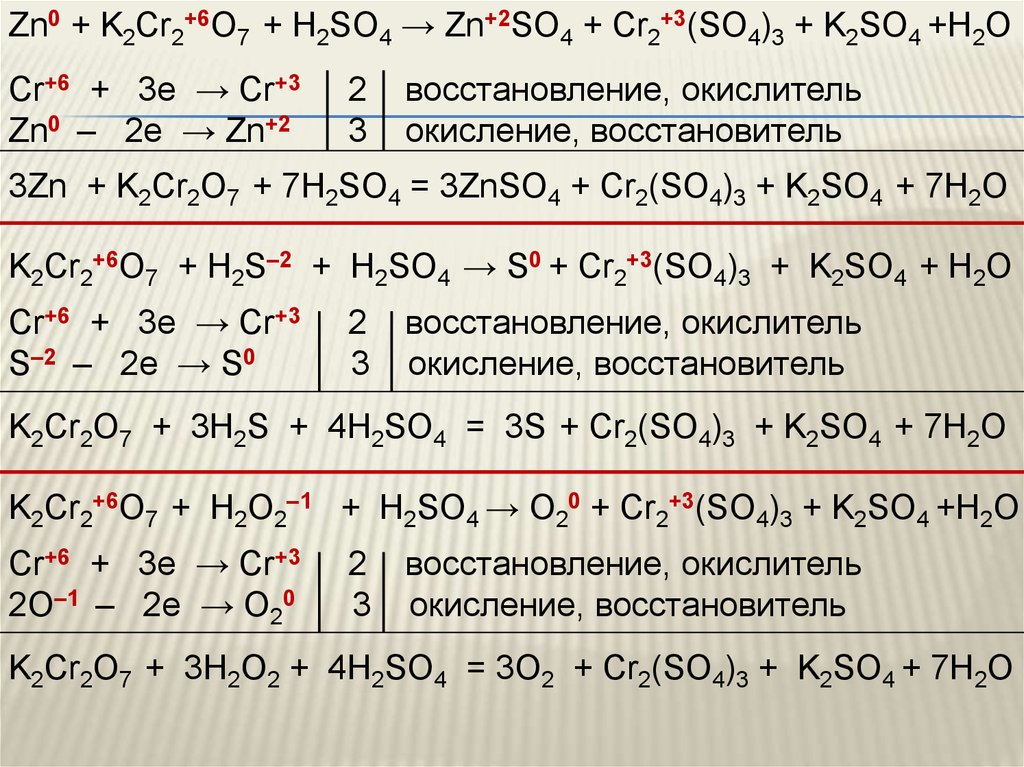
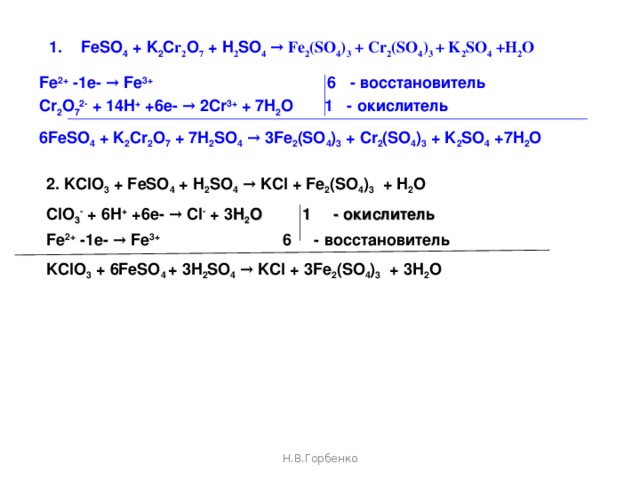
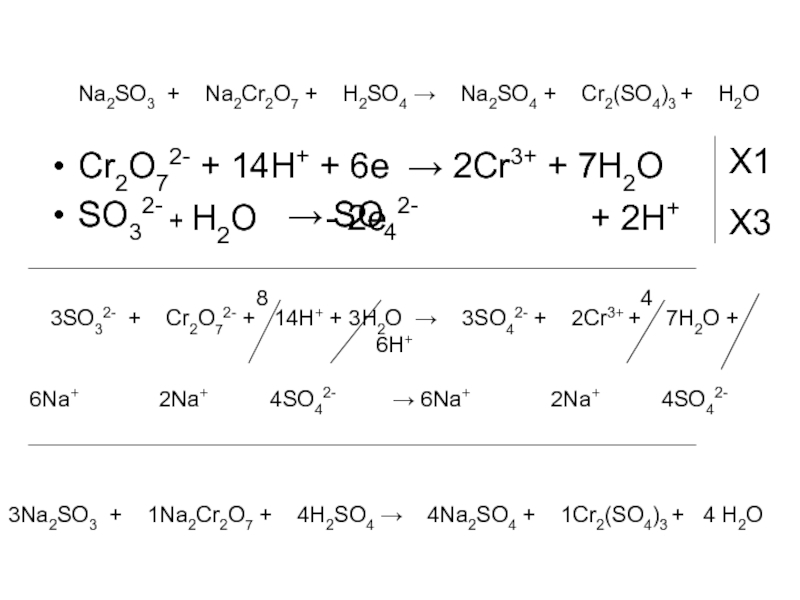
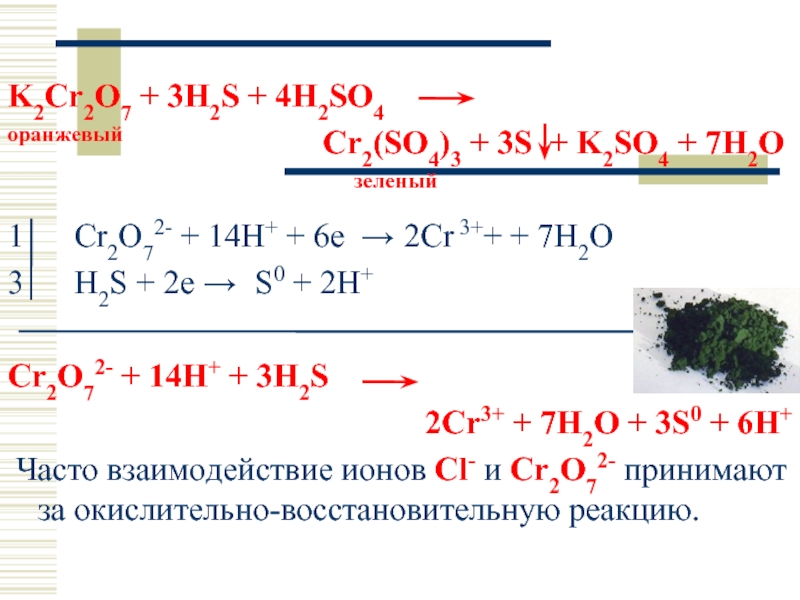
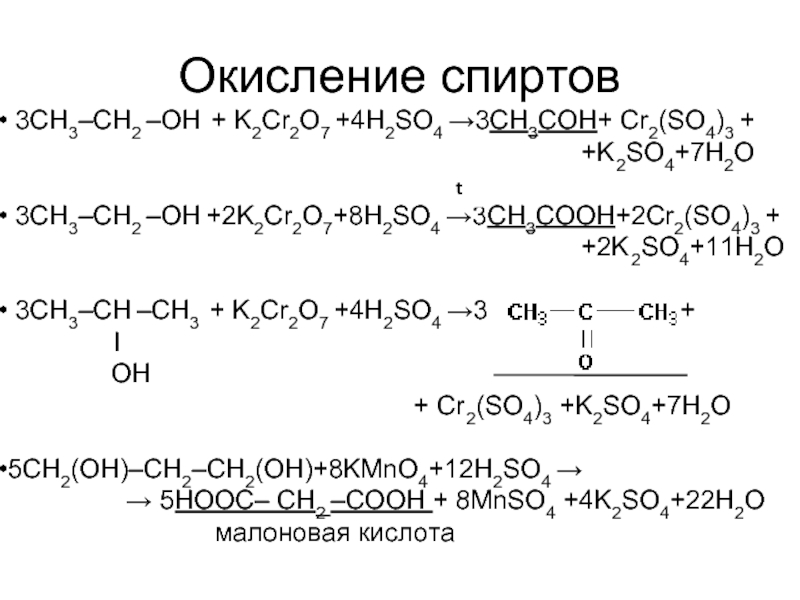
Решенное и коэффициентами уравнение реакции c2h5oh + 2 k2cr2o7 + 8 h2so4 → 2 co2 + 2 k2so4 + 2 cr2(so4)3 + 11 h2o с дополненными продуктами. Приложение для вычисления и …
Решенное и коэффициентами уравнение реакции c2h5oh + 2 k2cr2o7 + 8 h2so4 → 2 co2 + 2 k2so4 + 2 cr2(so4)3 + 11 h2o с дополненными продуктами. Приложение для вычисления и …
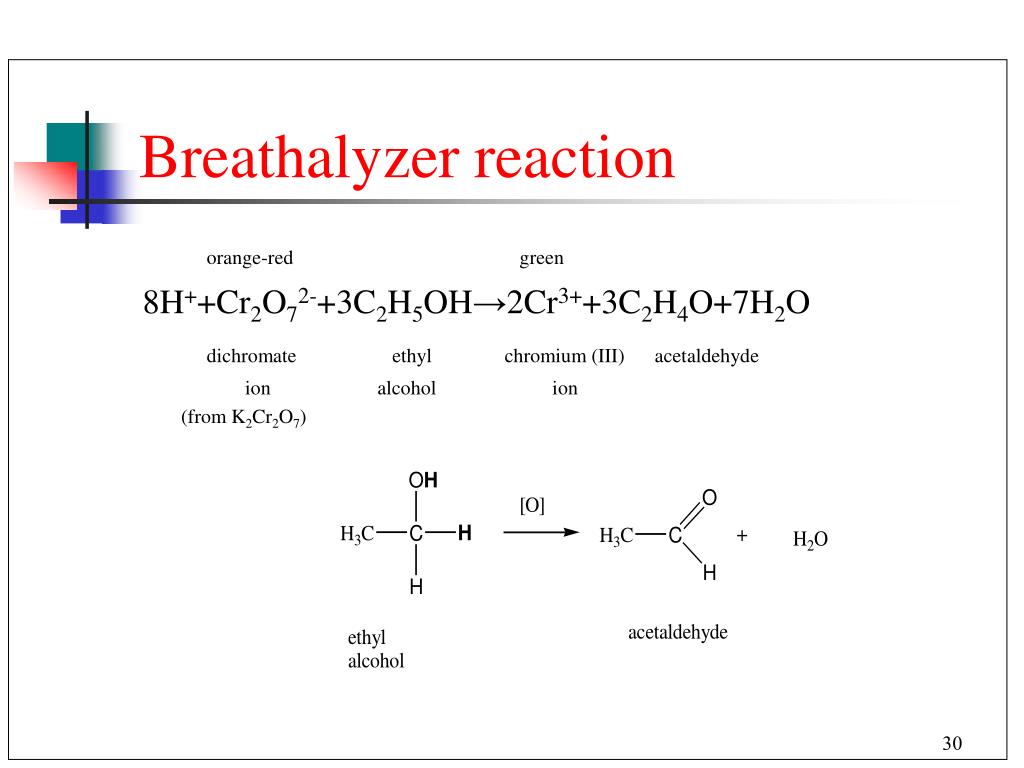
 This is an oxidation-reduction (redox) reaction: 3 C -II - 12 e - → 3 C II (oxidation) 4 Cr VI + 12 e - → 4 Cr III (reduction) C2H5OH is a reducing agent, K2Cr2O7 is an oxidizing agent. Reactants: …
This is an oxidation-reduction (redox) reaction: 3 C -II - 12 e - → 3 C II (oxidation) 4 Cr VI + 12 e - → 4 Cr III (reduction) C2H5OH is a reducing agent, K2Cr2O7 is an oxidizing agent. Reactants: …
 Balance the reaction of C2H5OH + K2Cr2O7 + H2SO4 = CH3COOH + Cr2(SO4)3 + K2SO4 + H2O using this chemical equation balancer!
Balance the reaction of C2H5OH + K2Cr2O7 + H2SO4 = CH3COOH + Cr2(SO4)3 + K2SO4 + H2O using this chemical equation balancer!
 A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
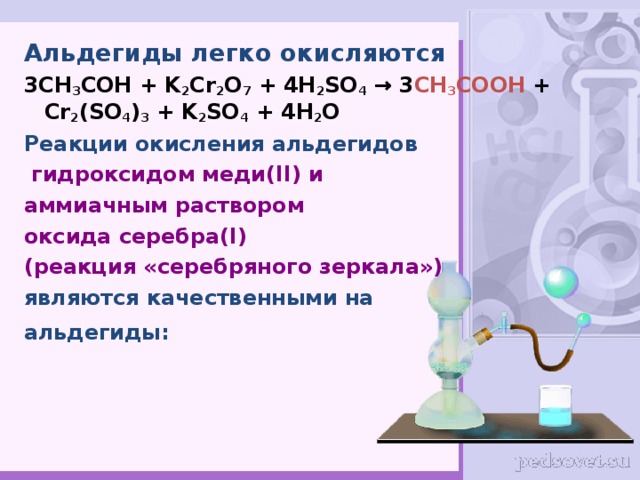
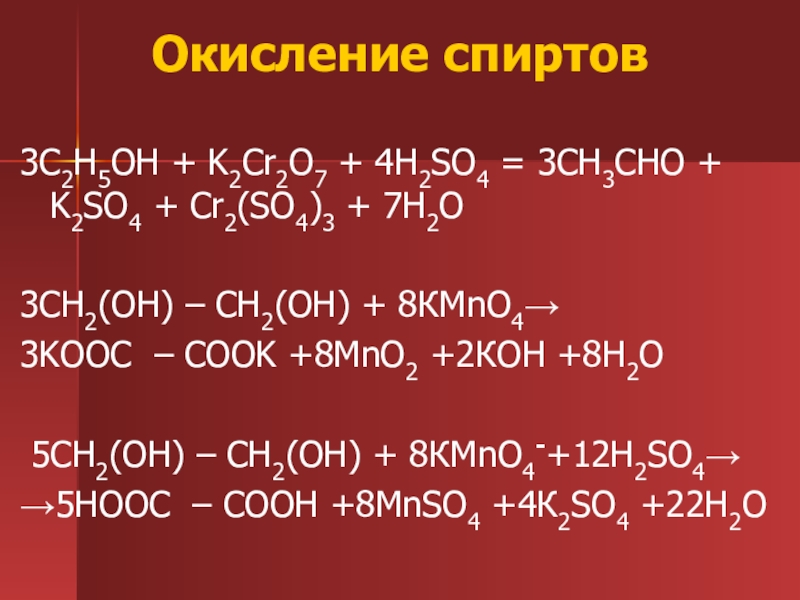
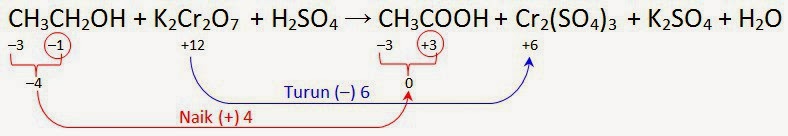
 C2H5OH является восстановителем, K2Cr2O7 является окислителем. Решенное и коэффициентами уравнение реакции 3 C2H5OH + 2 K2Cr2O7 + 8 H2SO4 → 3 CH3COOH …
C2H5OH является восстановителем, K2Cr2O7 является окислителем. Решенное и коэффициентами уравнение реакции 3 C2H5OH + 2 K2Cr2O7 + 8 H2SO4 → 3 CH3COOH …
 Cân bằng phương trình hay phản ứng hoá học C2H5OH + K2Cr2O7 + H2SO4 = CH3CHO + K2SO4 + Cr2(SO4)3 + H2O bằng cách sử dụng máy tính này!
Cân bằng phương trình hay phản ứng hoá học C2H5OH + K2Cr2O7 + H2SO4 = CH3CHO + K2SO4 + Cr2(SO4)3 + H2O bằng cách sử dụng máy tính này!
 Balance the reaction of C2H5OH + K2Cr2O7 + H2SO4 = CH3CHO + K2SO4 + Cr2(SO4)3 + H2O using this chemical equation balancer!
Balance the reaction of C2H5OH + K2Cr2O7 + H2SO4 = CH3CHO + K2SO4 + Cr2(SO4)3 + H2O using this chemical equation balancer!
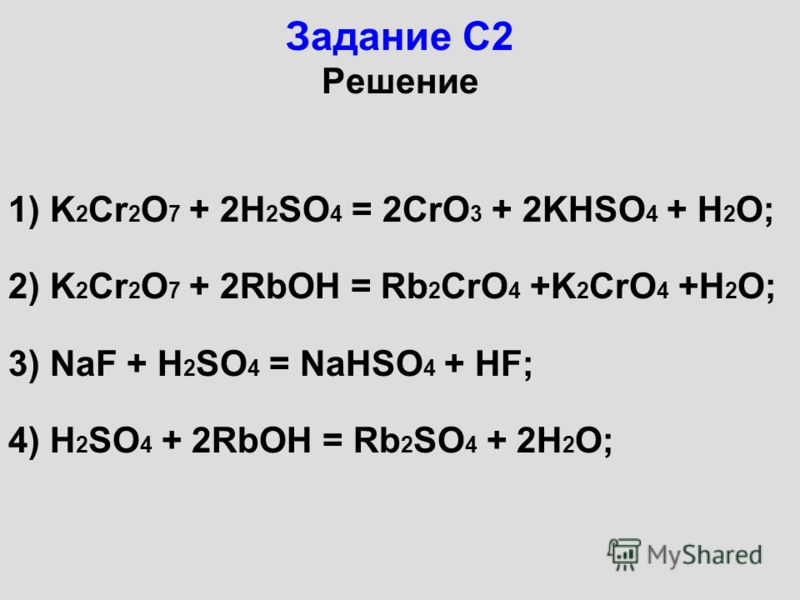
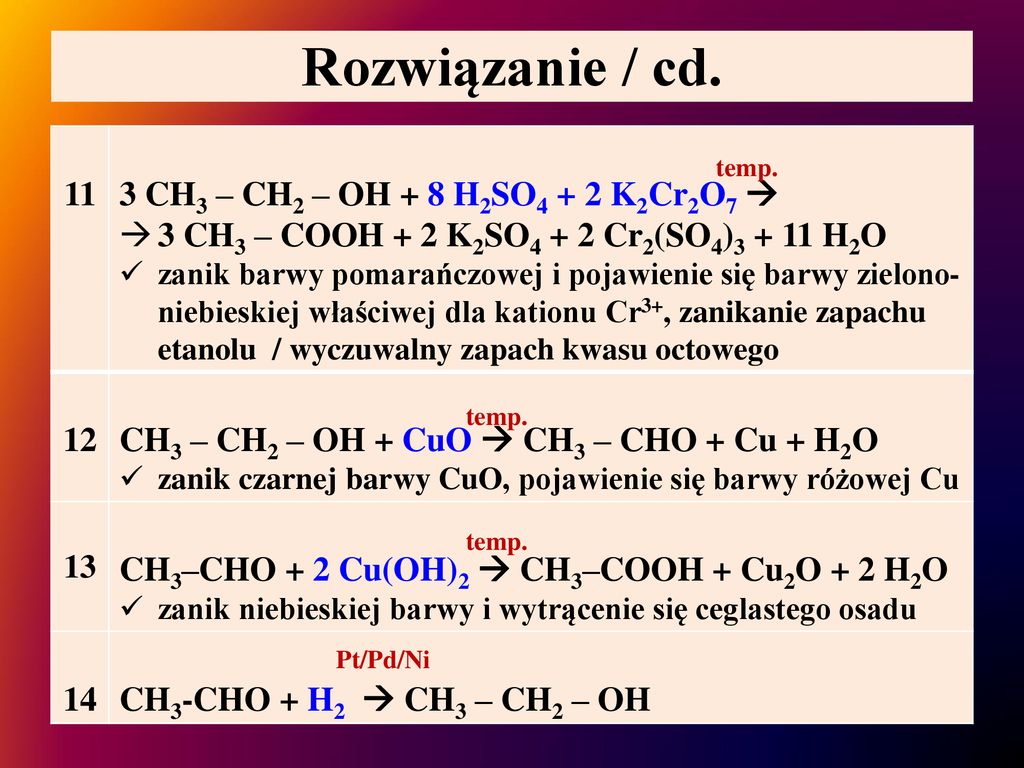 C2H5OH + K2Cr2O7 + H2SO4 = CH3COOH + K2SO4 + Cr2(SO4)3 + H2O Расставить коэффициенты методом полуреа - ответ на этот и другие вопросы получите онлайн на …
C2H5OH + K2Cr2O7 + H2SO4 = CH3COOH + K2SO4 + Cr2(SO4)3 + H2O Расставить коэффициенты методом полуреа - ответ на этот и другие вопросы получите онлайн на …
 a C 2 H 5 OH + b K 2 Cr 2 O 7 + c H 2 SO 4 = d CH 3 COOH + e CrS 2 O 8 + f K 2 SO 4 + g H 2 O Теперь запишем алгебраические уравнения баланса каждого атома:
a C 2 H 5 OH + b K 2 Cr 2 O 7 + c H 2 SO 4 = d CH 3 COOH + e CrS 2 O 8 + f K 2 SO 4 + g H 2 O Теперь запишем алгебраические уравнения баланса каждого атома:
 A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
 Решенное и коэффициентами уравнение реакции 3 C2H5OH + K2Cr2O7 + 4 H2SO4 → Cr2 (SO4)3 + 3 CH3CHO + K2SO4 + 7 H2O с дополненными продуктами.
Решенное и коэффициентами уравнение реакции 3 C2H5OH + K2Cr2O7 + 4 H2SO4 → Cr2 (SO4)3 + 3 CH3CHO + K2SO4 + 7 H2O с дополненными продуктами.
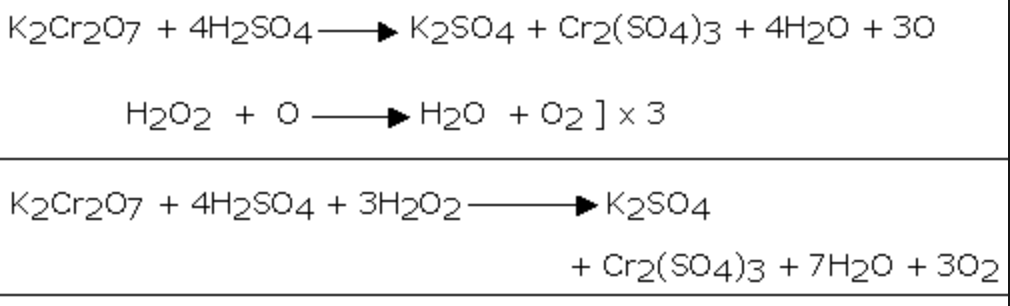
 3 c 2 h 5 oh + 2 k 2 cr 2 o 7 + 8 h 2 so 4 = 3 hc 2 h 3 o 2 + 2 cr 2 s 3 o 12 + 2 k 2 so 4 + 11 h 2 o. Стехиометрия.
3 c 2 h 5 oh + 2 k 2 cr 2 o 7 + 8 h 2 so 4 = 3 hc 2 h 3 o 2 + 2 cr 2 s 3 o 12 + 2 k 2 so 4 + 11 h 2 o. Стехиометрия.
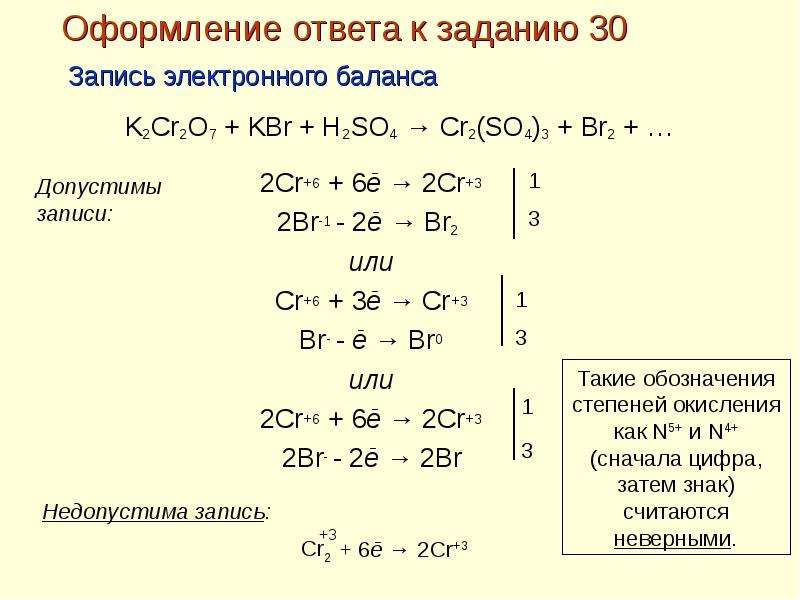
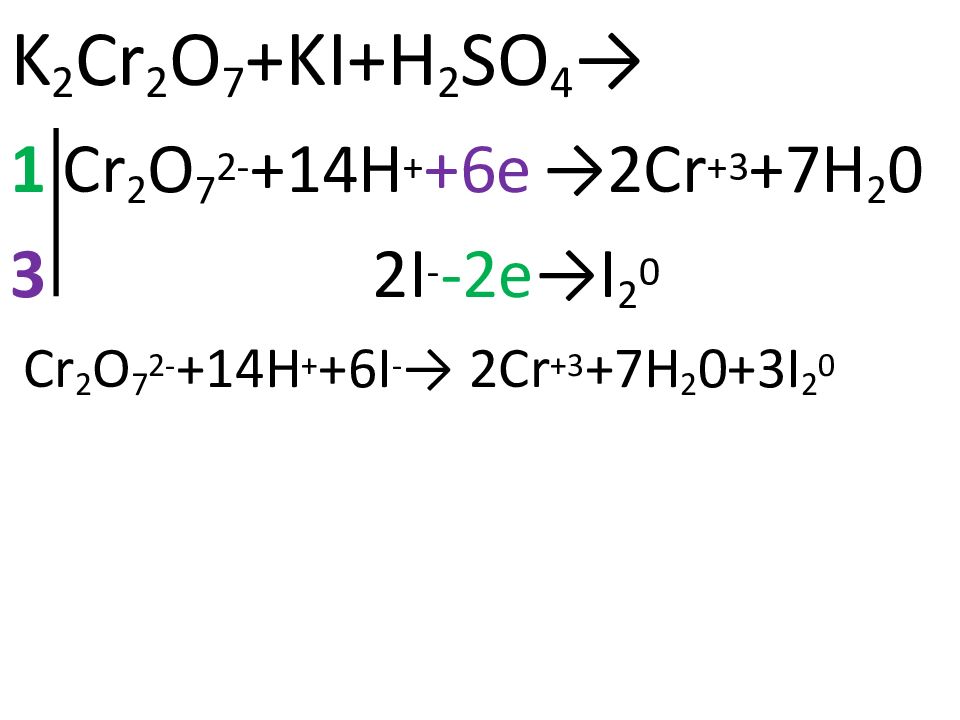 Balance the reaction of K2Cr2O7 + H2SO4 + C2H5OH = Cr2(SO4)3 + K2SO4 + CH3COOH + H2O using this chemical equation balancer!
Balance the reaction of K2Cr2O7 + H2SO4 + C2H5OH = Cr2(SO4)3 + K2SO4 + CH3COOH + H2O using this chemical equation balancer!
 Write the balanced equation for potassium bicarbonate reacting with sulfuric acid. Write a balanced chemical equation for the standard formation reaction of solid potassium dichromate …
Write the balanced equation for potassium bicarbonate reacting with sulfuric acid. Write a balanced chemical equation for the standard formation reaction of solid potassium dichromate …
 C2H5OH is a reducing agent, C2H5OH is an oxidizing agent, K2Cr2O7 is an oxidizing agent. Reactants: C2H5OH – Ethanol. Other names: Absolute alcohol , Alcohol , Cologne spirit..
C2H5OH is a reducing agent, C2H5OH is an oxidizing agent, K2Cr2O7 is an oxidizing agent. Reactants: C2H5OH – Ethanol. Other names: Absolute alcohol , Alcohol , Cologne spirit..
Еще по теме:

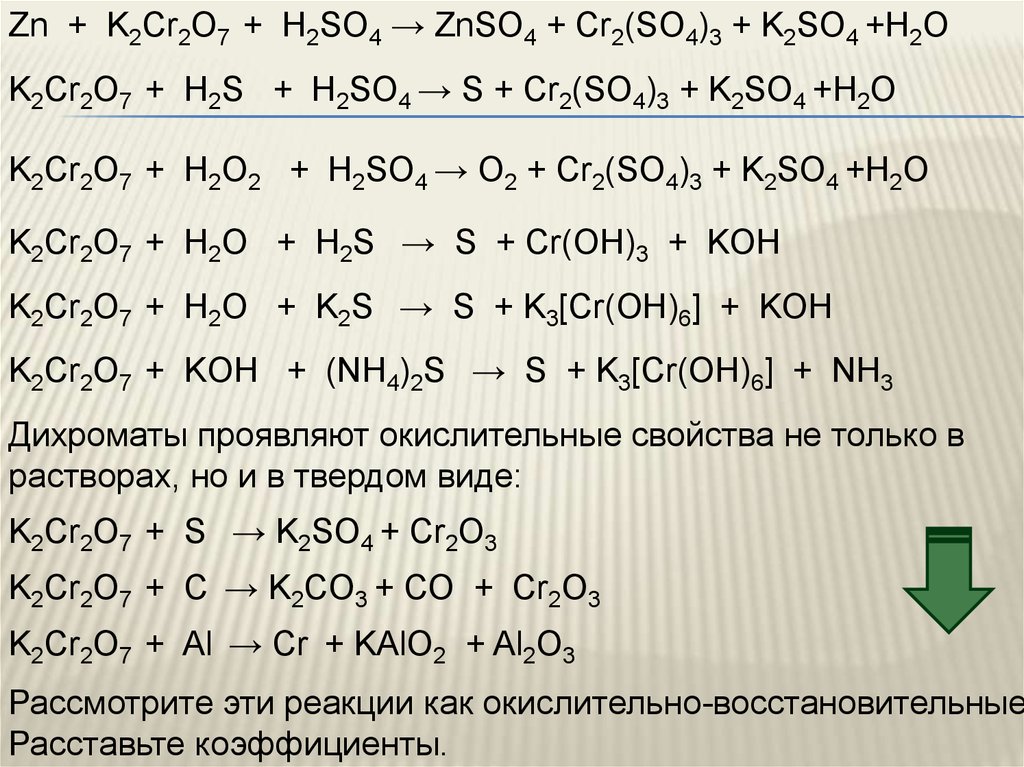







Еще по теме: