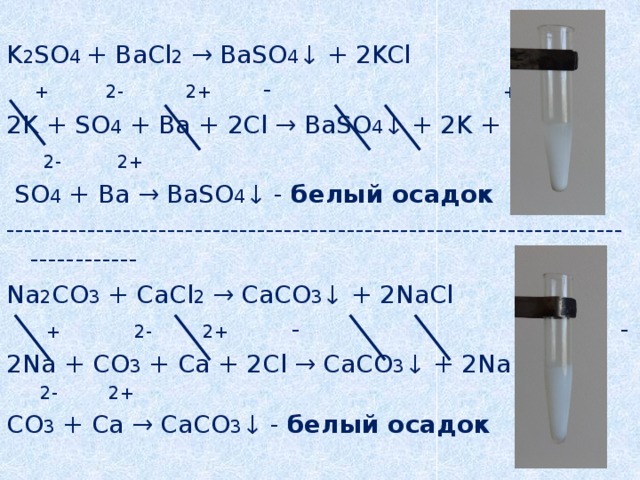
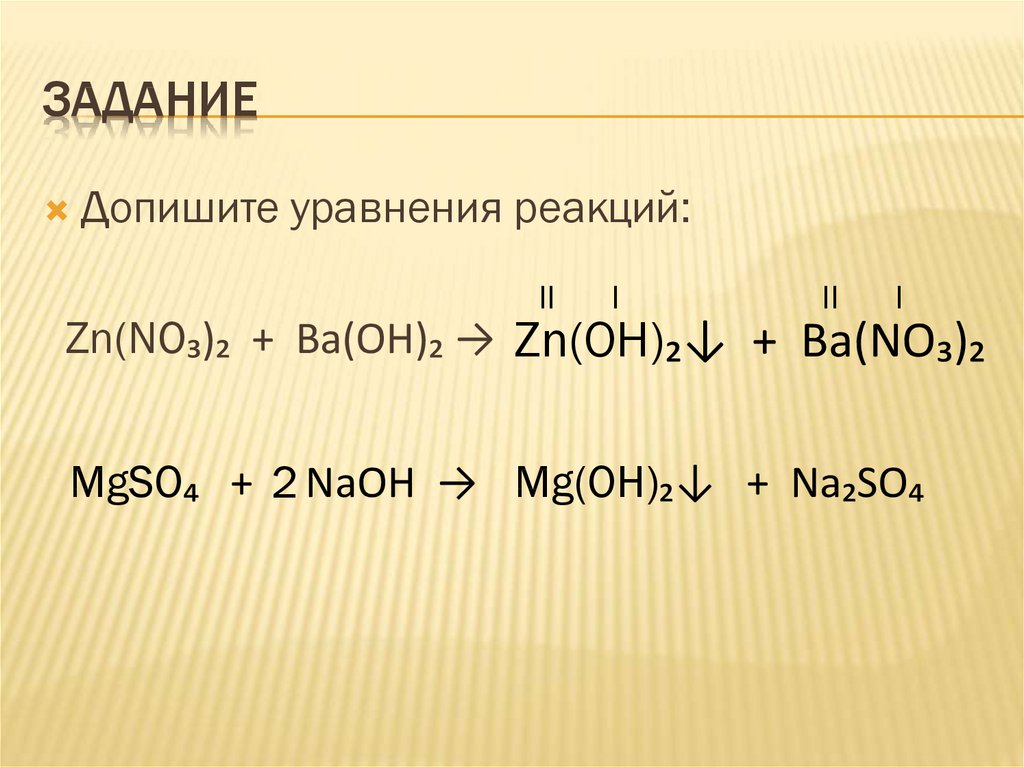
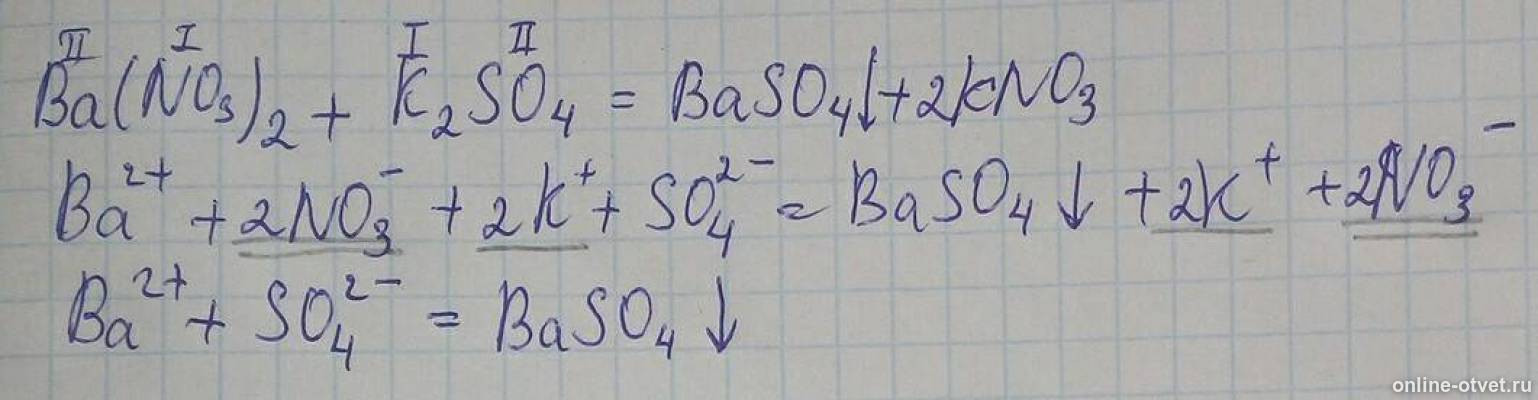 Решенное и коэффициентами уравнение реакции k2so4 + ba(no3)2 → 2 kno3 + baso4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции k2so4 + ba(no3)2 → 2 kno3 + baso4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Ba (NO3)2 + K2SO4 = BaSO4 + KNO3 is a Double Displacement (Metathesis) reaction where one mole of aqueous Barium Nitrate [Ba (NO 3) 2] and one mole of aqueous Potassium …
Ba (NO3)2 + K2SO4 = BaSO4 + KNO3 is a Double Displacement (Metathesis) reaction where one mole of aqueous Barium Nitrate [Ba (NO 3) 2] and one mole of aqueous Potassium …
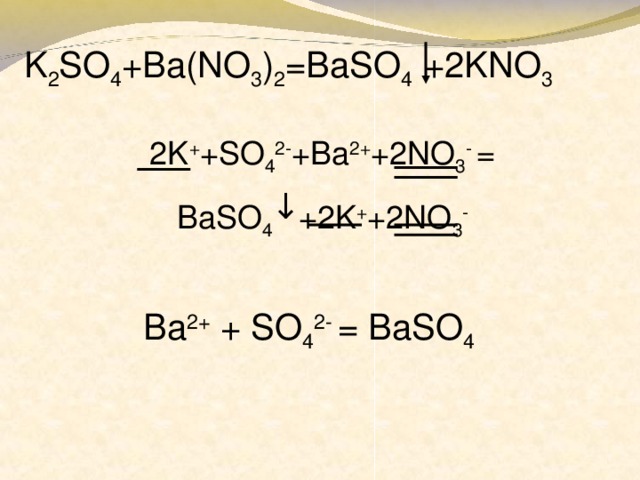 There are three main steps for writing the net ionic equation for K2SO4 + Ba (NO3)2 = BaSO4 + KNO3 (Potassium sulfate + Barium nitrate).
There are three main steps for writing the net ionic equation for K2SO4 + Ba (NO3)2 = BaSO4 + KNO3 (Potassium sulfate + Barium nitrate).
 Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 Ba (NO 3) 2 + 1 K 2 SO 4 = 1 BaSO 4 + 1 KNO 3. For each element, we check if the number of …
Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 Ba (NO 3) 2 + 1 K 2 SO 4 = 1 BaSO 4 + 1 KNO 3. For each element, we check if the number of …
 Решенное и коэффициентами уравнение реакции ba(no3)2 + k2so4 → baso4 + 2 kno3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции ba(no3)2 + k2so4 → baso4 + 2 kno3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
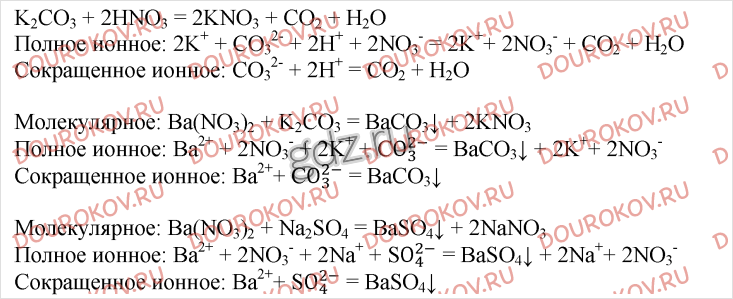 Ba (NO3)2+K2SO4 = 2KNO3 + BaSO4. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals.
Ba (NO3)2+K2SO4 = 2KNO3 + BaSO4. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals.
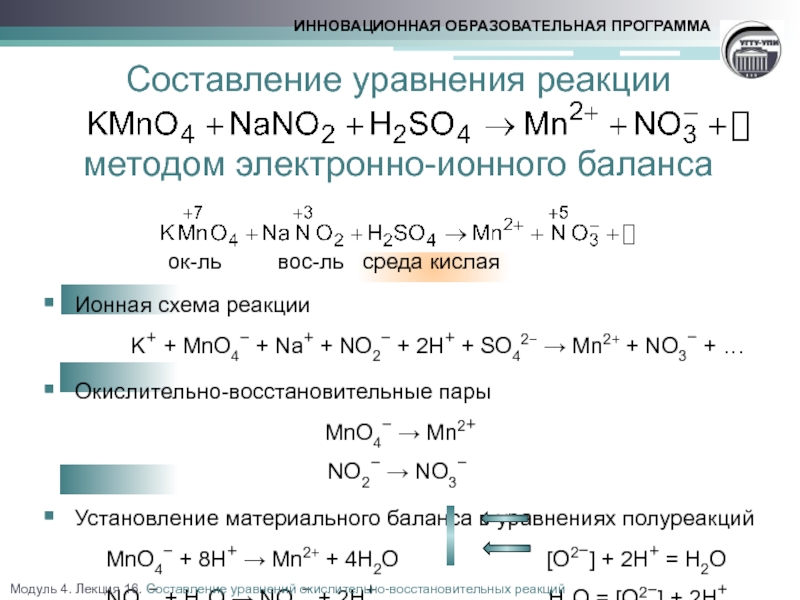
 Construct the equilibrium constant, K, expression for: K_2SO_4 + Ba(NO_3)_2 KNO_3 + BaSO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the activity expression for each chemical …
Construct the equilibrium constant, K, expression for: K_2SO_4 + Ba(NO_3)_2 KNO_3 + BaSO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the activity expression for each chemical …
 Ba (NO3)2 + K2SO4 = KNO3 + BaSO4 is a Double Displacement (Metathesis) reaction where one mole of aqueous Barium Nitrate [Ba (NO 3) 2] and one mole of aqueous Potassium …
Ba (NO3)2 + K2SO4 = KNO3 + BaSO4 is a Double Displacement (Metathesis) reaction where one mole of aqueous Barium Nitrate [Ba (NO 3) 2] and one mole of aqueous Potassium …
 Ba(NO3)2 + K2SO4 -> BaSO4 + 2KNO3. Nitrate (NO3-) salts are generally soluble in water, so potassium nitrate is actually present as K+ and NO3- ions. Therefore, the precipitate formed is …
Ba(NO3)2 + K2SO4 -> BaSO4 + 2KNO3. Nitrate (NO3-) salts are generally soluble in water, so potassium nitrate is actually present as K+ and NO3- ions. Therefore, the precipitate formed is …
 BaSO4 + KNO3 = Ba(NO3)2 + K2SO4 is a Double Displacement (Metathesis) reaction where one mole of solid Barium Sulfate [BaSO 4] and two moles of aqueous Potassium Nitrate [KNO 3] …
BaSO4 + KNO3 = Ba(NO3)2 + K2SO4 is a Double Displacement (Metathesis) reaction where one mole of solid Barium Sulfate [BaSO 4] and two moles of aqueous Potassium Nitrate [KNO 3] …
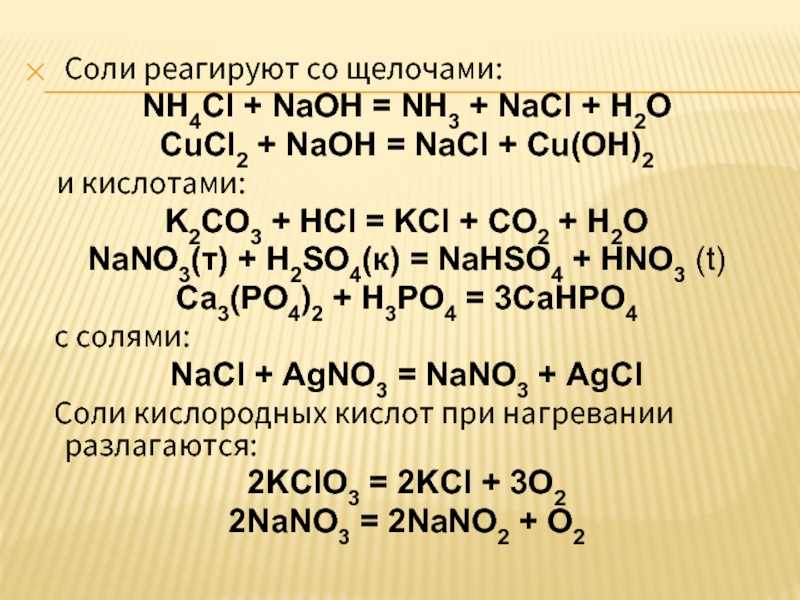
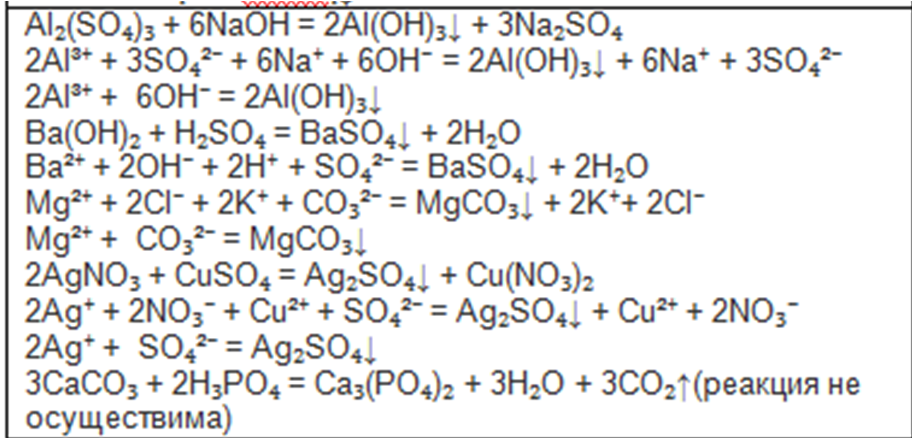 ba(no3)2+k2so4 → baso4+kno3. 2 Решите задачу. Для консервирования овощей необходимо приготовить 6%-й раствор поваренной соли. Вычислите массу соли и воды, которые …
ba(no3)2+k2so4 → baso4+kno3. 2 Решите задачу. Для консервирования овощей необходимо приготовить 6%-й раствор поваренной соли. Вычислите массу соли и воды, которые …
 1 ba(no 3) 2 + 1 k 2 so 4 = 1 kno 3 + 1 baso 4 For each element, we check if the number of atoms is balanced on both sides of the equation. Ba is balanced: 1 atom in reagents and 1 atom in …
1 ba(no 3) 2 + 1 k 2 so 4 = 1 kno 3 + 1 baso 4 For each element, we check if the number of atoms is balanced on both sides of the equation. Ba is balanced: 1 atom in reagents and 1 atom in …
 K+ (aq) + SO4-2 (aq) --> KSO4 (s) Ba+ (aq) + Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.
K+ (aq) + SO4-2 (aq) --> KSO4 (s) Ba+ (aq) + Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.
 K2SO4 + Ba(NO3)2 = BaSO4 + KNO3 is a Double Displacement (Metathesis) reaction where one mole of aqueous Potassium Sulfate [K 2 SO 4] and one mole of aqueous Barium Nitrate …
K2SO4 + Ba(NO3)2 = BaSO4 + KNO3 is a Double Displacement (Metathesis) reaction where one mole of aqueous Potassium Sulfate [K 2 SO 4] and one mole of aqueous Barium Nitrate …
 1 ba(no 3) 2 + 1 k 2 so 4 = 1 (ba) 2 (so 4) 2 + 1 kno 3 For each element, we check if the number of atoms is balanced on both sides of the equation. Ba is not balanced: 1 atom in reagents and 2 …
1 ba(no 3) 2 + 1 k 2 so 4 = 1 (ba) 2 (so 4) 2 + 1 kno 3 For each element, we check if the number of atoms is balanced on both sides of the equation. Ba is not balanced: 1 atom in reagents and 2 …
Еще по теме:
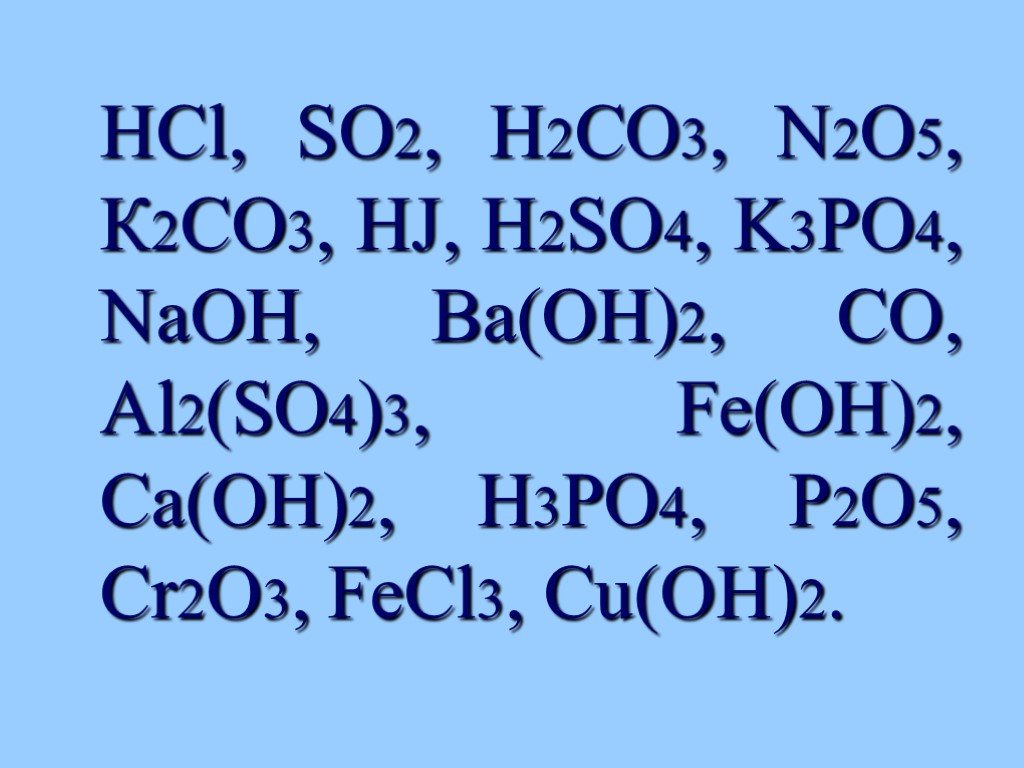












Еще по теме: