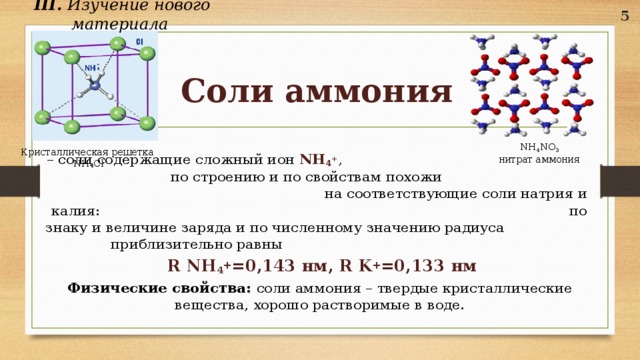
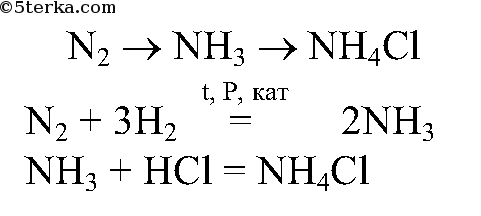Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Its other major … See more
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Its other major … See more
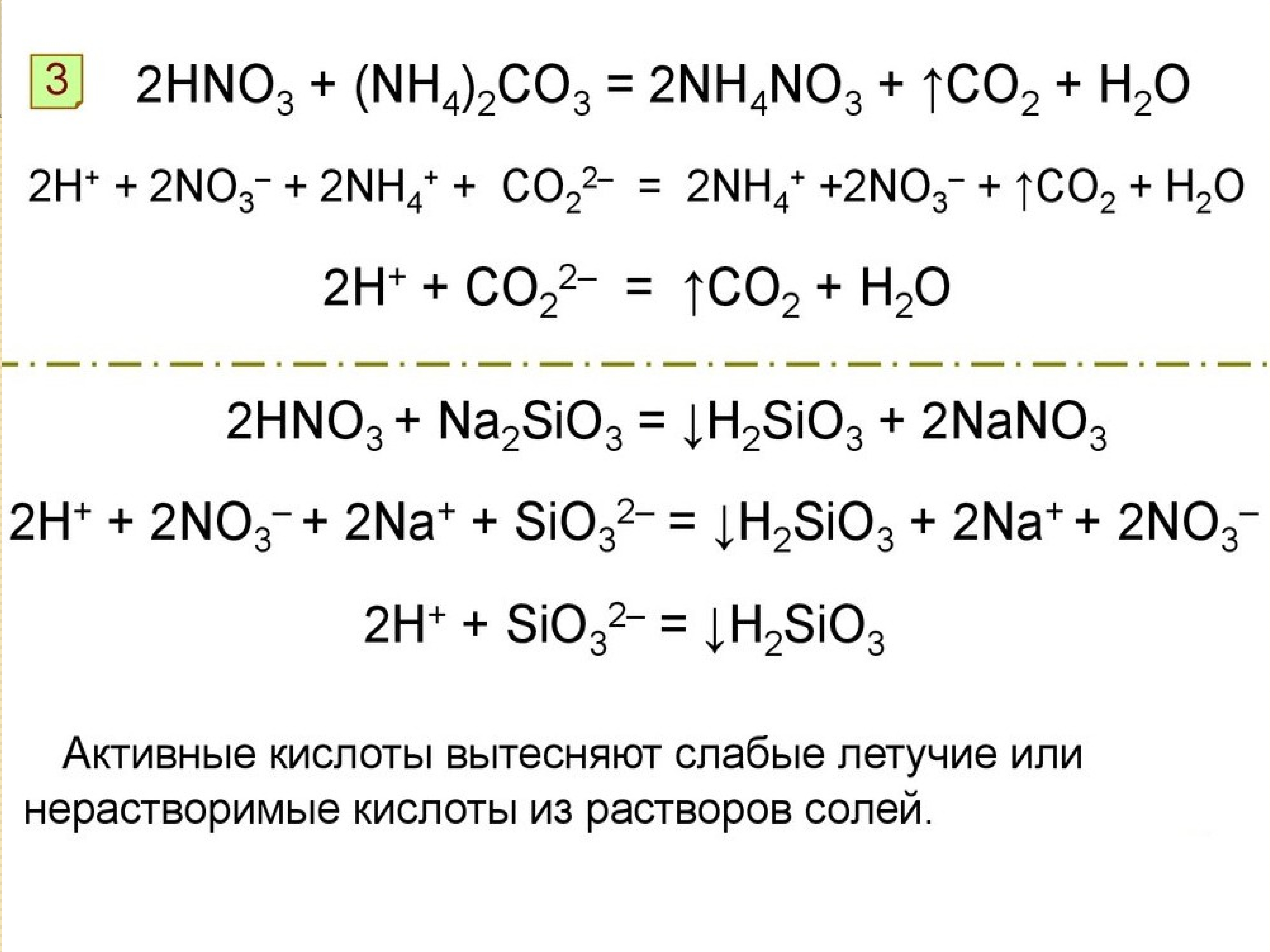
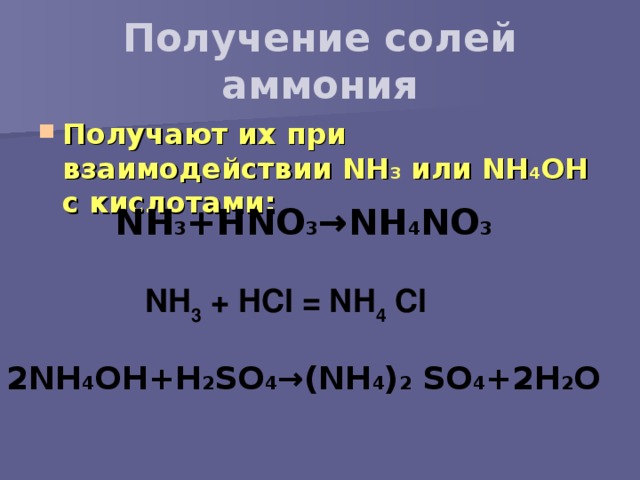 NH3(aq)+HNO3(aq)→NH4NO3(aq) Это кислотно-щелочная реакция (нейтрализа́ция): NH3 является щелочным, HNO3 представляет собой кислоту. Реактанты: NH3. Названия: …
NH3(aq)+HNO3(aq)→NH4NO3(aq) Это кислотно-щелочная реакция (нейтрализа́ция): NH3 является щелочным, HNO3 представляет собой кислоту. Реактанты: NH3. Названия: …
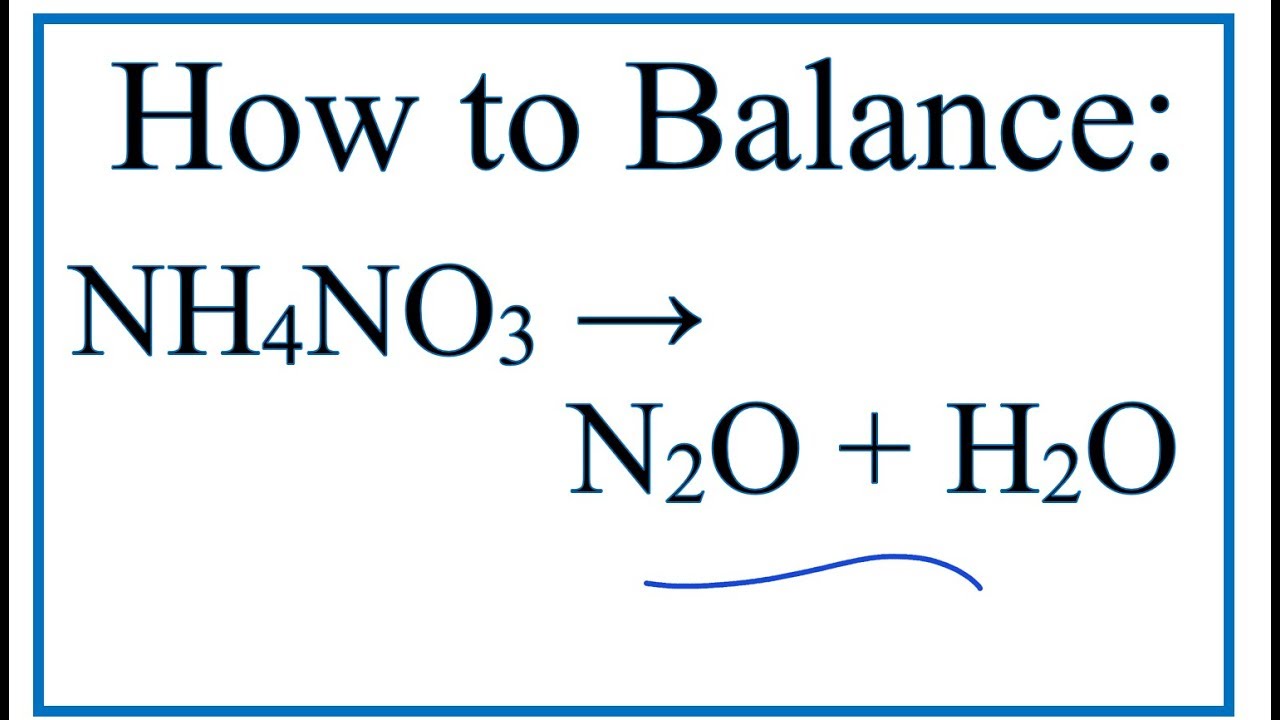
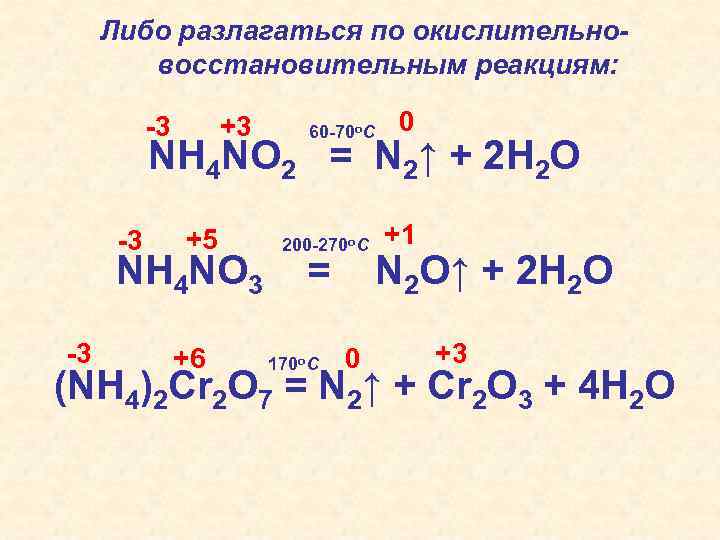
 NH4NO3 = NH3 + HNO3 is a Decomposition reaction where one mole of Ammonium Nitrate [NH 4 NO 3] decomposes into one mole of Ammonia [NH 3] and one mole of Nitric Acid [HNO 3]
NH4NO3 = NH3 + HNO3 is a Decomposition reaction where one mole of Ammonium Nitrate [NH 4 NO 3] decomposes into one mole of Ammonia [NH 3] and one mole of Nitric Acid [HNO 3]
 Ammonium nitrate (NH4NO3) is produced by neutralizing nitric acid (HNO3) with ammonia (NH3).. All ammonium nitrate plants produce an aqueous ammonium nitrate solution through the …
Ammonium nitrate (NH4NO3) is produced by neutralizing nitric acid (HNO3) with ammonia (NH3).. All ammonium nitrate plants produce an aqueous ammonium nitrate solution through the …
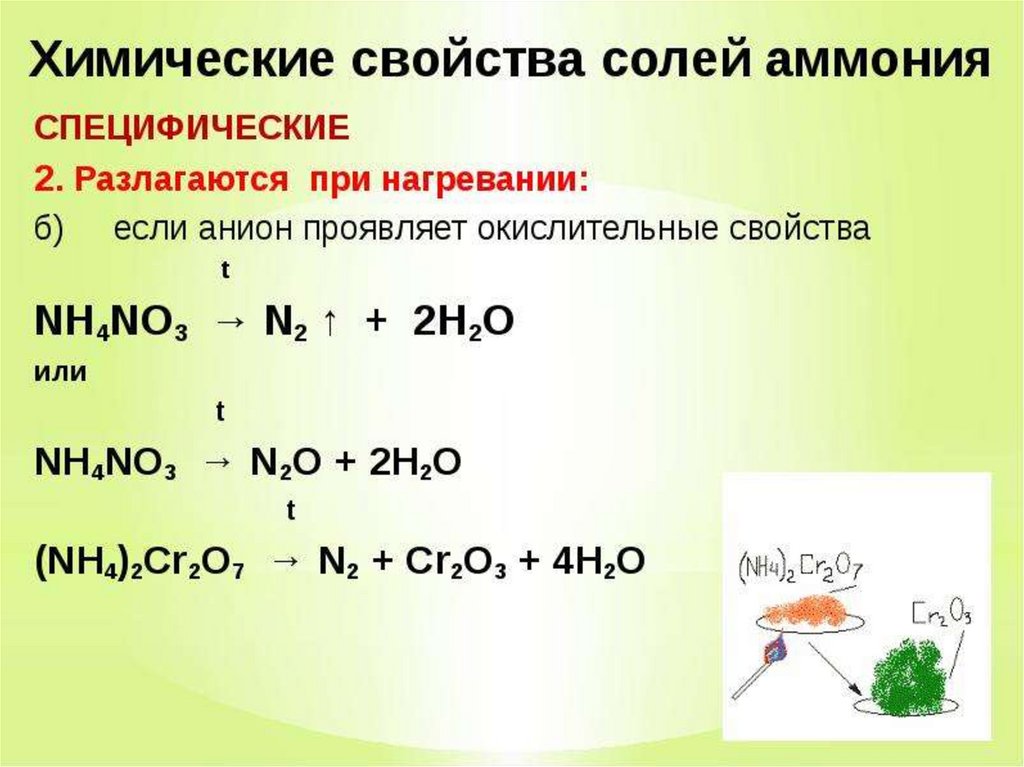
 NH 4 NO 3 can be prepared from the acid-base reaction between nitric acid and ammonia, described by the following chemical equation: NH3 + HNO3 → NH4NO3. This reaction is highly exothermic and proceeds violently. …
NH 4 NO 3 can be prepared from the acid-base reaction between nitric acid and ammonia, described by the following chemical equation: NH3 + HNO3 → NH4NO3. This reaction is highly exothermic and proceeds violently. …
 All atoms are now balanced and the whole equation is fully balanced: NH 3 + HNO 3 = NH 4 NO 3. Balancing step by step using the algebraic method. Let's balance this equation using the …
All atoms are now balanced and the whole equation is fully balanced: NH 3 + HNO 3 = NH 4 NO 3. Balancing step by step using the algebraic method. Let's balance this equation using the …
 ammonium nitrate, (NH 4 NO 3), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. The commercial grade contains about 33.5 percent nitrogen, all of …
ammonium nitrate, (NH 4 NO 3), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. The commercial grade contains about 33.5 percent nitrogen, all of …
 In this video we'll balance the equation NH3 + HNO3 = NH4NO3 and provide the correct coefficients for each compound.To balance NH3 + HNO3 = NH4NO3 you'll nee.
In this video we'll balance the equation NH3 + HNO3 = NH4NO3 and provide the correct coefficients for each compound.To balance NH3 + HNO3 = NH4NO3 you'll nee.
 There are three main steps for writing the net ionic equation for NH3 + HNO3 = NH4NO3 (Ammonia + Nitric acid).
There are three main steps for writing the net ionic equation for NH3 + HNO3 = NH4NO3 (Ammonia + Nitric acid).
 Bài viết này sẽ giải thích chi tiết về quá trình tạo ra NH3 từ NH4NO3, các điều kiện cần thiết và ứng dụng thực tiễn trong sản xuất công nghiệp, nông nghiệp cũng như những lưu ý an toàn khi sử dụng.
Bài viết này sẽ giải thích chi tiết về quá trình tạo ra NH3 từ NH4NO3, các điều kiện cần thiết và ứng dụng thực tiễn trong sản xuất công nghiệp, nông nghiệp cũng như những lưu ý an toàn khi sử dụng.
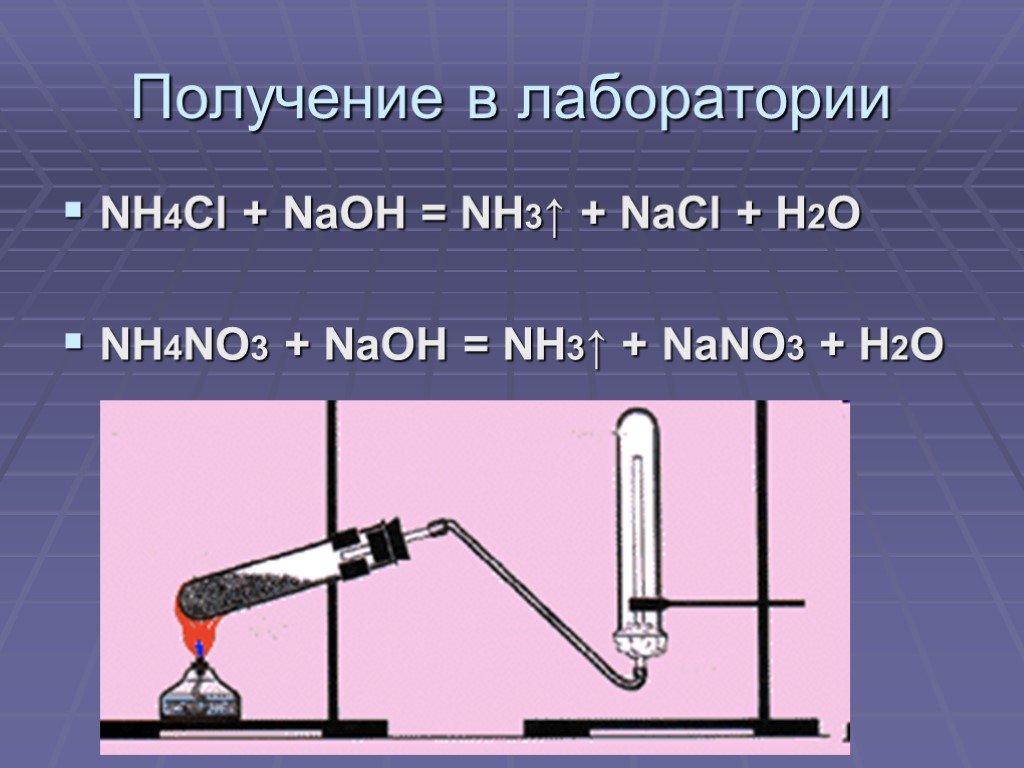
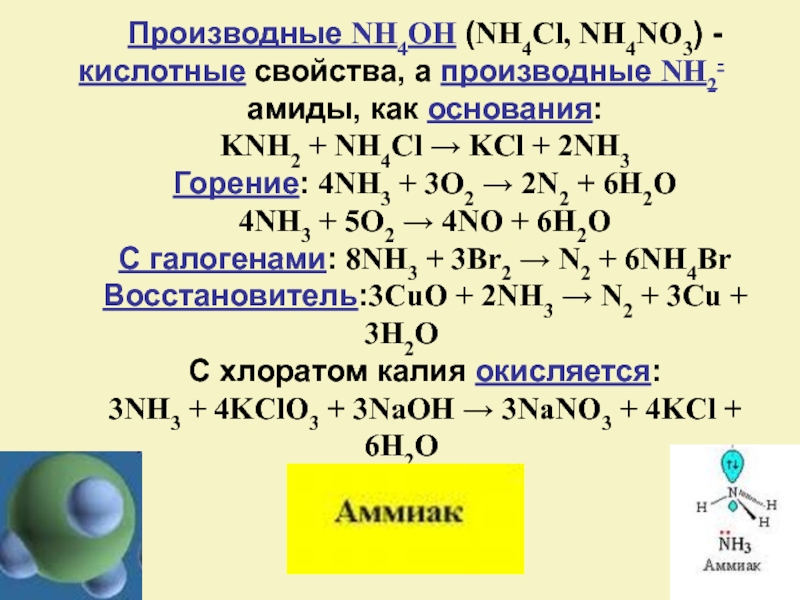
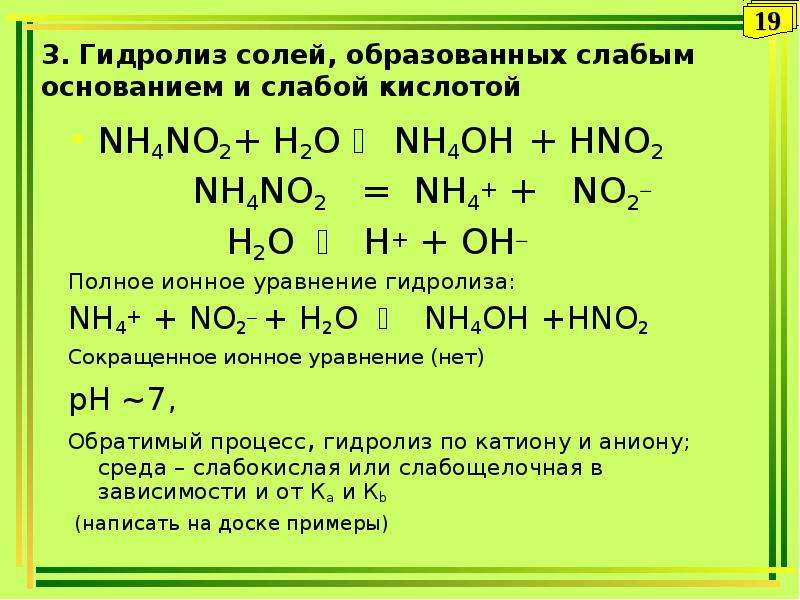
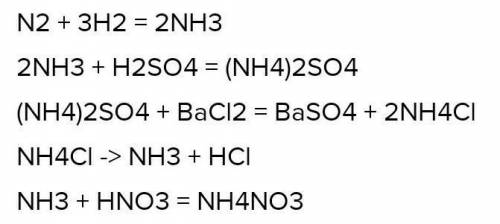
 Любая соль аммония при нагревании (в сухом виде) со щёлочью выделяет газообразный аммиак: nh4no3 + koh (нагрев) = kno3 + h2o + nh3
Любая соль аммония при нагревании (в сухом виде) со щёлочью выделяет газообразный аммиак: nh4no3 + koh (нагрев) = kno3 + h2o + nh3
 NH3 + HNO3 = NH4NO3 is a Synthesis reaction where one mole of Ammonia [NH 3] and one mole of Nitric Acid [HNO 3] combine to form one mole of Ammonium Nitrate [NH 4 NO 3]
NH3 + HNO3 = NH4NO3 is a Synthesis reaction where one mole of Ammonia [NH 3] and one mole of Nitric Acid [HNO 3] combine to form one mole of Ammonium Nitrate [NH 4 NO 3]
 I thought that the acid $\ce{HNO3}$ would just give its hydrogen to $\ce{NH3}$ and make the resulting reaction: $$\ce{NH_3 + HNO_3 -> HNH_3 + NO_3}$$ However the correct answer is …
I thought that the acid $\ce{HNO3}$ would just give its hydrogen to $\ce{NH3}$ and make the resulting reaction: $$\ce{NH_3 + HNO_3 -> HNH_3 + NO_3}$$ However the correct answer is …
 HNO3 + NH3 = NH4NO3 is a Synthesis reaction where one mole of Nitric Acid [HNO 3] and one mole of Ammonia [NH 3] combine to form one mole of Ammonium Nitrate [NH 4 NO 3]
HNO3 + NH3 = NH4NO3 is a Synthesis reaction where one mole of Nitric Acid [HNO 3] and one mole of Ammonia [NH 3] combine to form one mole of Ammonium Nitrate [NH 4 NO 3]
 Внимание правильный ответ: NH4NO3 + NaOH (конц. ) = NaNO3 + NH3•H2O (При нагревании) При нагревании до 250-300 градусов пойдет вот такая "интересная" реакция …
Внимание правильный ответ: NH4NO3 + NaOH (конц. ) = NaNO3 + NH3•H2O (При нагревании) При нагревании до 250-300 градусов пойдет вот такая "интересная" реакция …
Еще по теме:

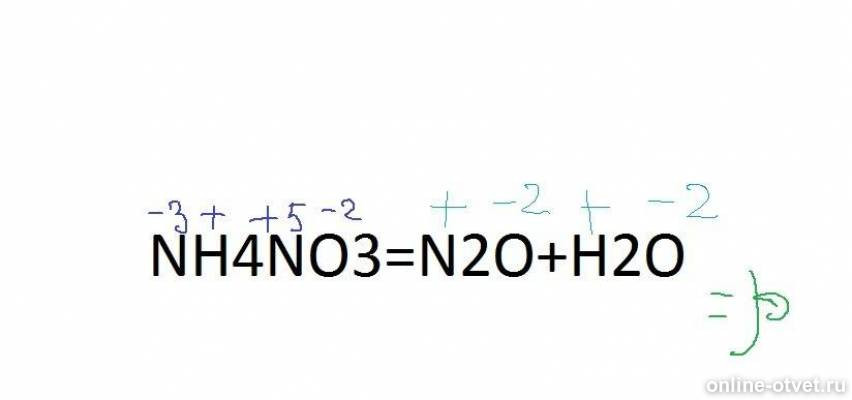








Еще по теме: