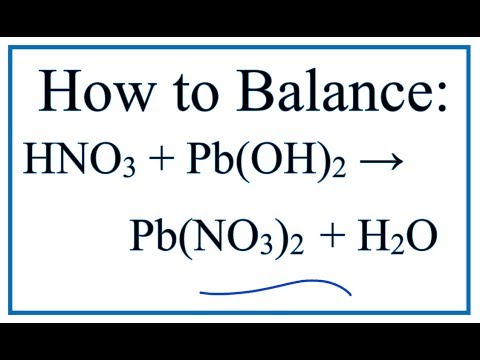
Решенное и коэффициентами уравнение реакции 2 HCl + Pb(NO3)2 → 2 HNO3 + PbCl2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
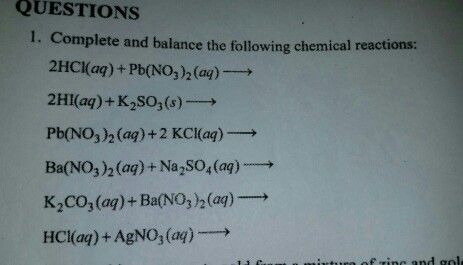
Решенное и коэффициентами уравнение реакции 2 HCl + Pb(NO3)2 → 2 HNO3 + PbCl2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Это кислотно-щелочная реакция (нейтрализа́ция): Pb (N O 3) 2 является щелочным, H Cl представляет собой кислоту. Это реакция осаждения : Pb Cl 2 сформированный …
Это кислотно-щелочная реакция (нейтрализа́ция): Pb (N O 3) 2 является щелочным, H Cl представляет собой кислоту. Это реакция осаждения : Pb Cl 2 сформированный …
 Pb(NO3)2 + HCl = Pb(Cl)2 + HNO3 is a Double Displacement (Metathesis) reaction where one mole of aqueous Lead(Ii) Nitrate [Pb(NO 3) 2] and two moles of aqueous Hydrogen Chloride …
Pb(NO3)2 + HCl = Pb(Cl)2 + HNO3 is a Double Displacement (Metathesis) reaction where one mole of aqueous Lead(Ii) Nitrate [Pb(NO 3) 2] and two moles of aqueous Hydrogen Chloride …
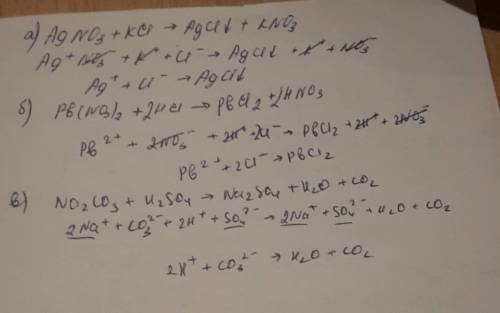
 1 Pb(No 3) 2 + 1 HCl = 1 PbCl 2 + 1 HNo 3 Для каждого элемента мы проверяем, сбалансировано ли количество атомов в обеих частях уравнения. Pb сбалансирован: 1 …
1 Pb(No 3) 2 + 1 HCl = 1 PbCl 2 + 1 HNo 3 Для каждого элемента мы проверяем, сбалансировано ли количество атомов в обеих частях уравнения. Pb сбалансирован: 1 …
 2HCl(aq)+Pb(NO3)2(aq)→2HNO3(aq)+PbCl2(s) This is an acid-base reaction ( neutralization ): HCl is an acid, Pb(NO3)2 is a base. This is a precipitation reaction: PbCl2 is the formed …
2HCl(aq)+Pb(NO3)2(aq)→2HNO3(aq)+PbCl2(s) This is an acid-base reaction ( neutralization ): HCl is an acid, Pb(NO3)2 is a base. This is a precipitation reaction: PbCl2 is the formed …
 Solution. When dilute Hydrochloric acid is added to Lead Nitrate solution, it gives a white precipitate of Lead chloride and Nitric acid. The chemical reaction is as follows: Pb (NO 3) 2 …
Solution. When dilute Hydrochloric acid is added to Lead Nitrate solution, it gives a white precipitate of Lead chloride and Nitric acid. The chemical reaction is as follows: Pb (NO 3) 2 …
 First, we set all coefficients to 1: 1 Pb (NO 3) 2 + 1 HCl = 1 PbCl 2 + 1 HNO 3. For each element, we check if the number of atoms is balanced on both sides of the equation. Pb is balanced: 1 …
First, we set all coefficients to 1: 1 Pb (NO 3) 2 + 1 HCl = 1 PbCl 2 + 1 HNO 3. For each element, we check if the number of atoms is balanced on both sides of the equation. Pb is balanced: 1 …
 Pb(NO3)2 + HCl = HNO3 + PbCl2 is a Double Displacement (Metathesis) reaction where one mole of aqueous Lead(Ii) Nitrate [Pb(NO 3) 2] and two moles of aqueous Hydrogen Chloride …
Pb(NO3)2 + HCl = HNO3 + PbCl2 is a Double Displacement (Metathesis) reaction where one mole of aqueous Lead(Ii) Nitrate [Pb(NO 3) 2] and two moles of aqueous Hydrogen Chloride …
 HCl + Pb (NO3)2 = PbCl2 + HNO3 is a Double Displacement (Metathesis) reaction where two moles of aqueous Hydrogen Chloride [HCl] and one mole of aqueous Lead (Ii) Nitrate [Pb (NO …
HCl + Pb (NO3)2 = PbCl2 + HNO3 is a Double Displacement (Metathesis) reaction where two moles of aqueous Hydrogen Chloride [HCl] and one mole of aqueous Lead (Ii) Nitrate [Pb (NO …
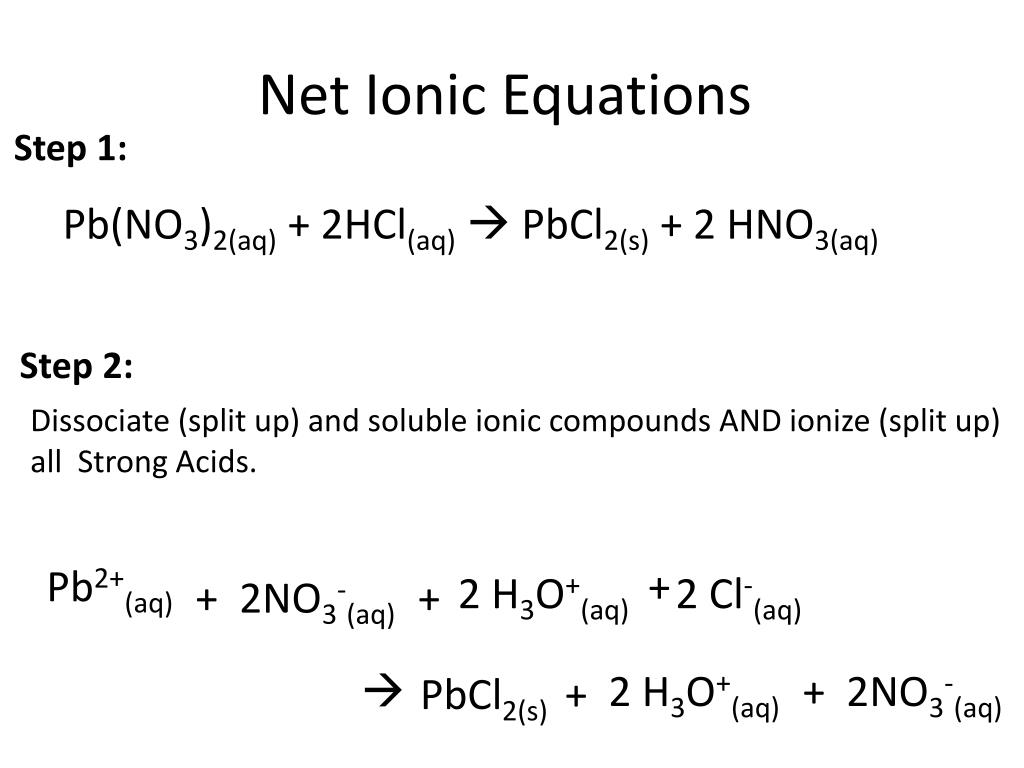
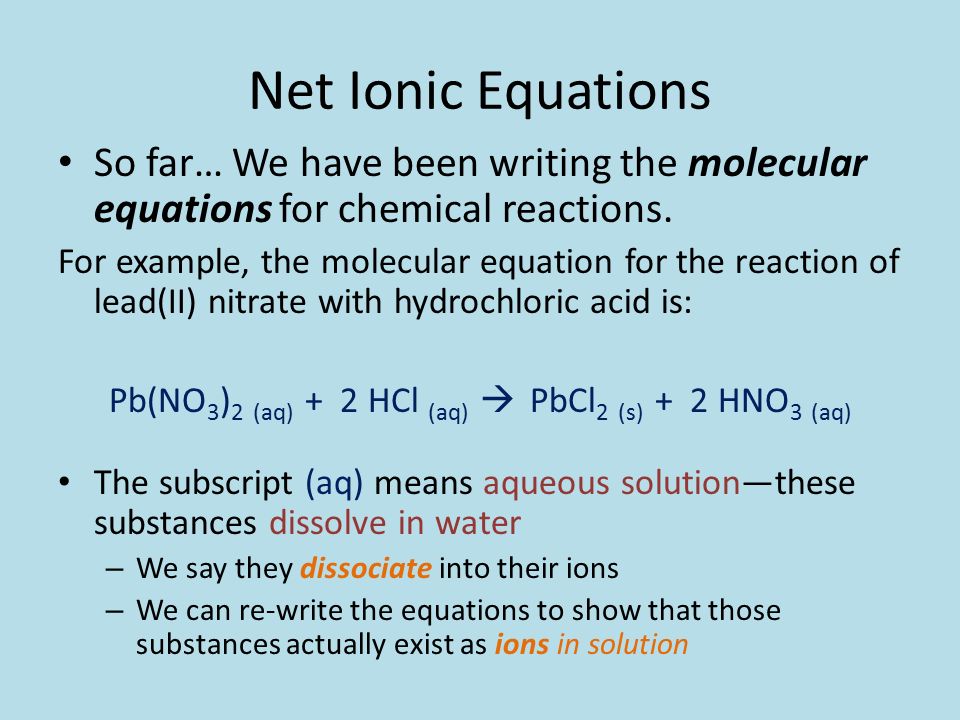
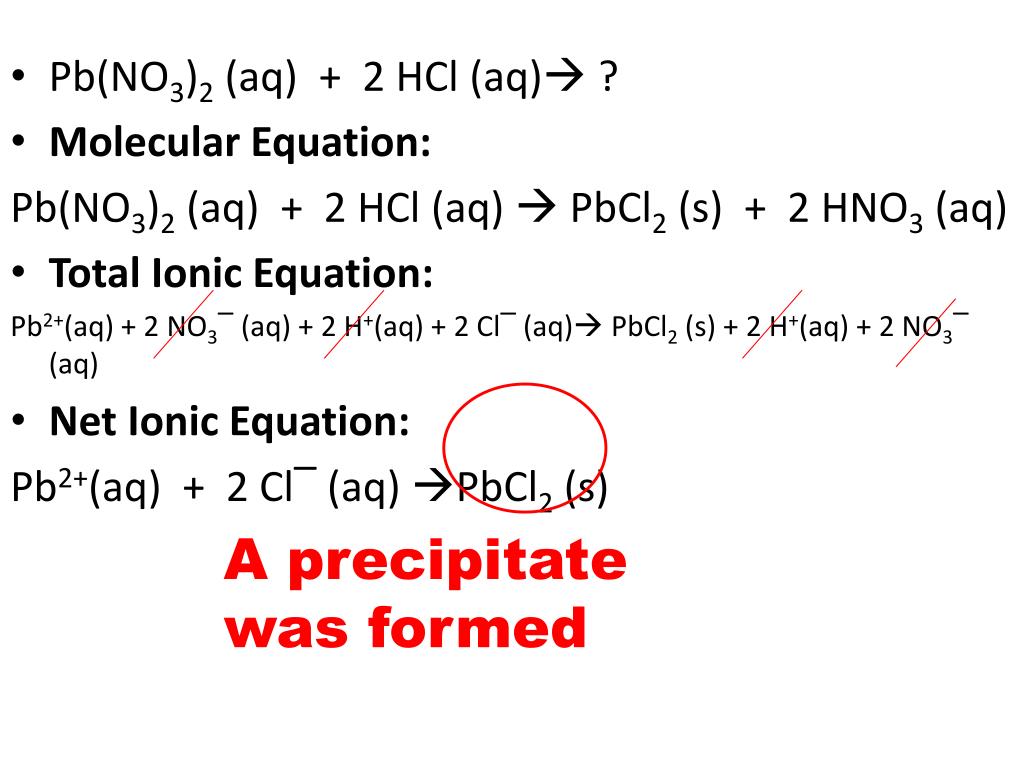
 There are three main steps for writing the net ionic equation for HCl + Pb (NO3)2 = PbCl2 + HNO3 (Hydrochloric acid + Lead (II) nitrate). First, we balance the molecular equation. Second,.
There are three main steps for writing the net ionic equation for HCl + Pb (NO3)2 = PbCl2 + HNO3 (Hydrochloric acid + Lead (II) nitrate). First, we balance the molecular equation. Second,.
 1 Pb(NO 3) 2 (aq) + 1 HCl(aq) = 1 PbCl 2 (s) + 1 HNO 3 (aq) For each element, we check if the number of atoms is balanced on both sides of the equation. Pb is balanced: 1 atom in reagents …
1 Pb(NO 3) 2 (aq) + 1 HCl(aq) = 1 PbCl 2 (s) + 1 HNO 3 (aq) For each element, we check if the number of atoms is balanced on both sides of the equation. Pb is balanced: 1 atom in reagents …
 Solved and balanced chemical equation Pb(NO3)2 + 2 HCl → 2 HNO3 + PbCl2 with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation Pb(NO3)2 + 2 HCl → 2 HNO3 + PbCl2 with completed products. Application for completing products and balancing equations.
 Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
 HCl + Pb(NO3)2 = HNO3 + PbCl2 is a Double Displacement (Metathesis) reaction where two moles of aqueous Hydrogen Chloride [HCl] and one mole of aqueous Lead(Ii) Nitrate [Pb(NO …
HCl + Pb(NO3)2 = HNO3 + PbCl2 is a Double Displacement (Metathesis) reaction where two moles of aqueous Hydrogen Chloride [HCl] and one mole of aqueous Lead(Ii) Nitrate [Pb(NO …
 Balance the reaction of Pb(NO3)2 + HCl = PbCl2 + NO3 + H2 using this chemical equation balancer!
Balance the reaction of Pb(NO3)2 + HCl = PbCl2 + NO3 + H2 using this chemical equation balancer!
Еще по теме:

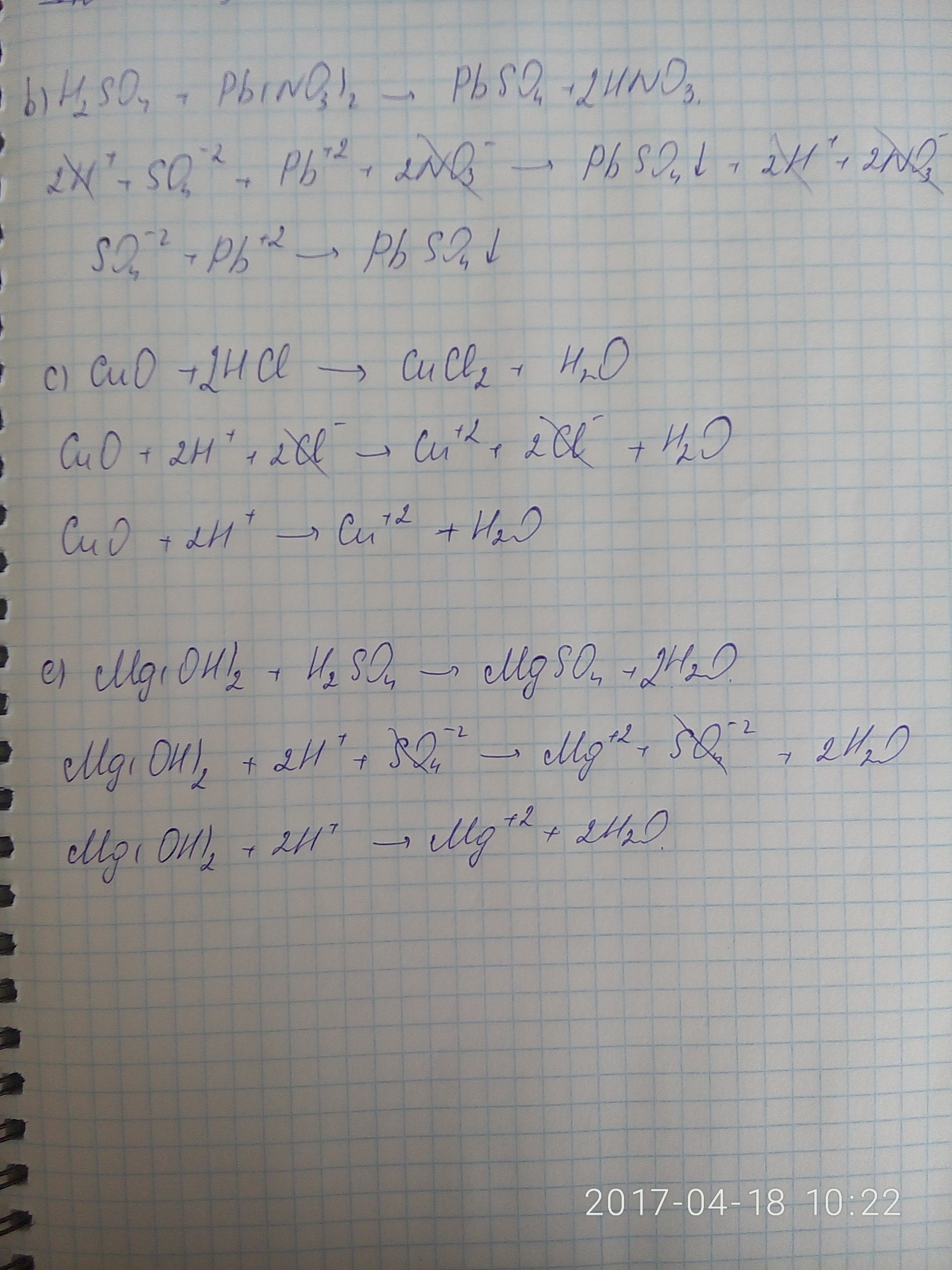








Еще по теме: