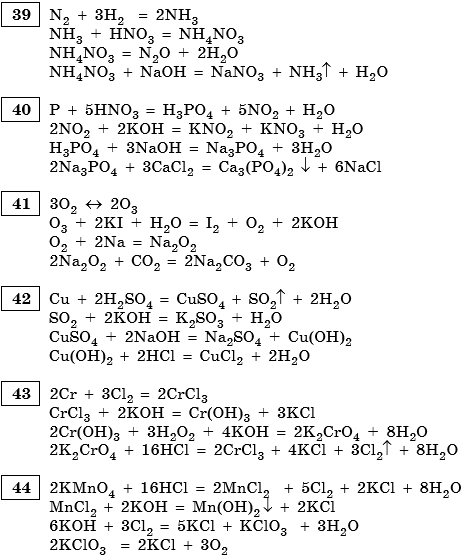
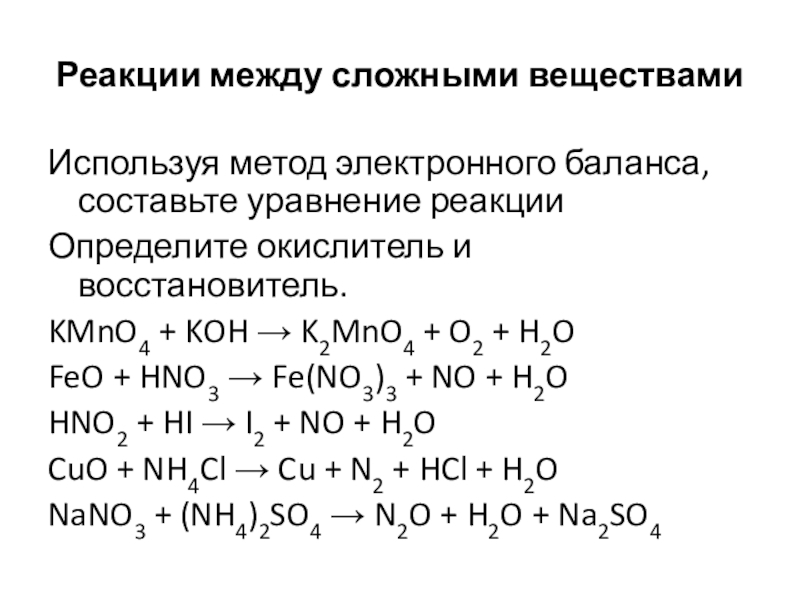
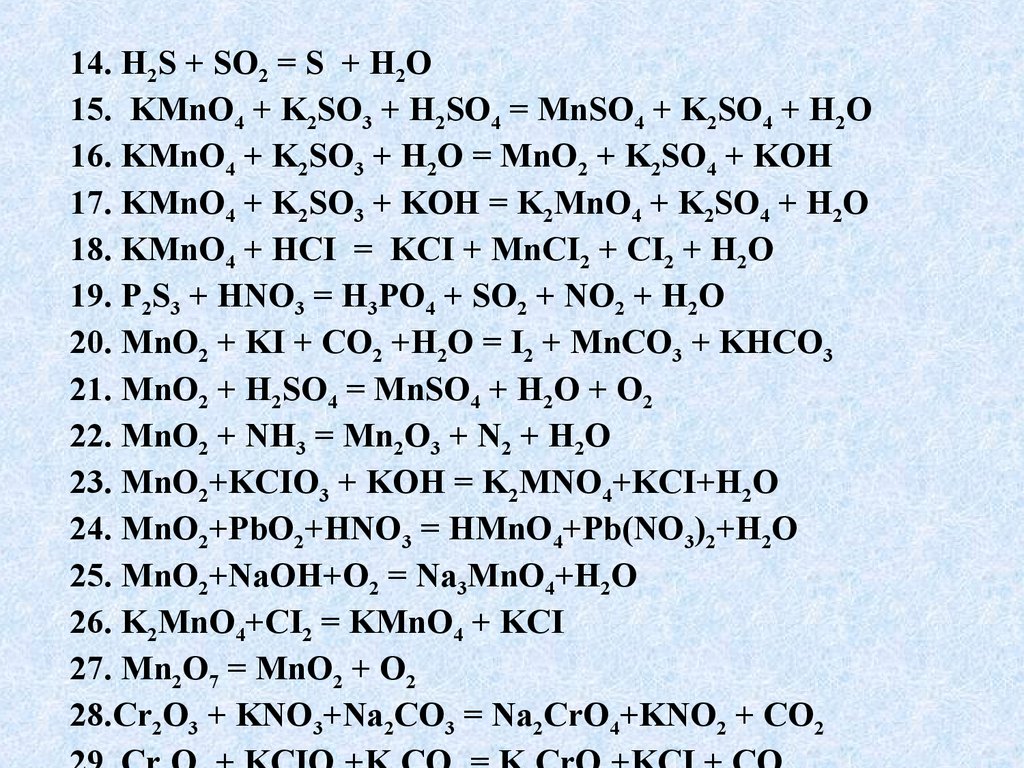
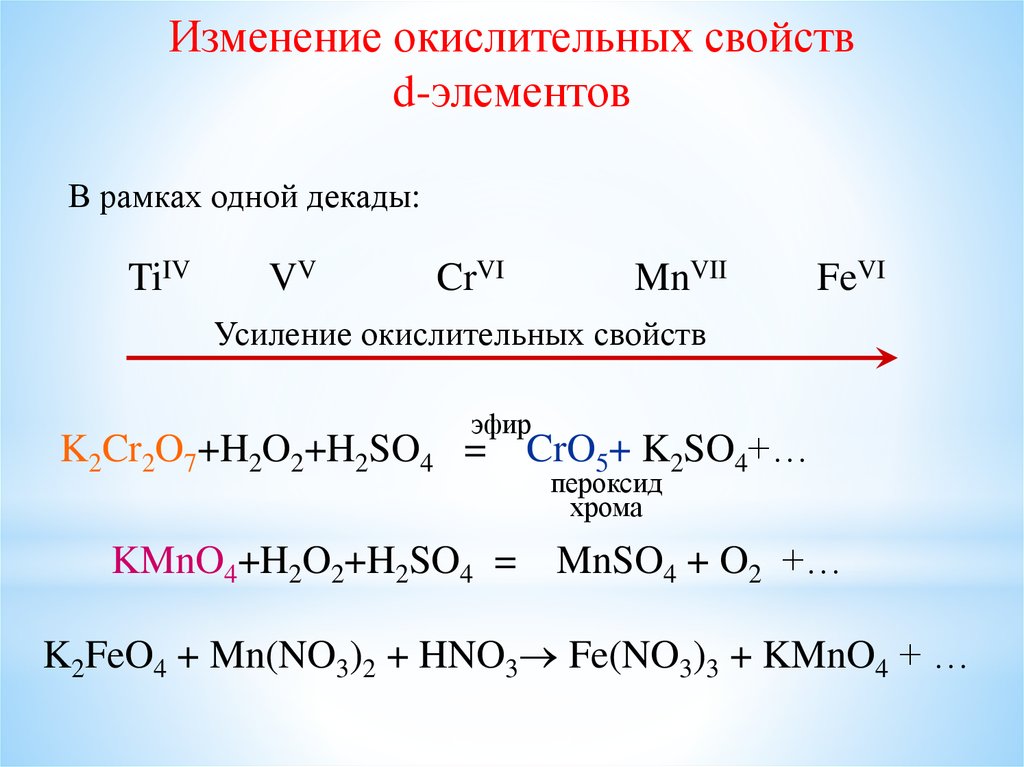
 Решенное и коэффициентами уравнение реакции kmno4 + 3 no2 + h2o → 2 hno3 + mno2 + kno3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции kmno4 + 3 no2 + h2o → 2 hno3 + mno2 + kno3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Balance the reaction of KMnO4 + HNO3 = KNO3 + MnO2 + H2 + O3 using this chemical equation balancer!
Balance the reaction of KMnO4 + HNO3 = KNO3 + MnO2 + H2 + O3 using this chemical equation balancer!
 KMnO4 + 3 NO2 + H2O → 2 HNO3 + MnO2 + KNO3 - Balanced equation | Chemical Equations online! KMnO4 is an oxidizing agent, NO2 is a reducing agent. Appearance: Brown gas ; …
KMnO4 + 3 NO2 + H2O → 2 HNO3 + MnO2 + KNO3 - Balanced equation | Chemical Equations online! KMnO4 is an oxidizing agent, NO2 is a reducing agent. Appearance: Brown gas ; …
 Решенное и коэффициентами уравнение реакции 3 Mn (NO3)2 + 2 KMnO4 + 2 H2O → 4 HNO3 + 5 MnO2 + 2 KNO3 с дополненными продуктами. Приложение для вычисления и …
Решенное и коэффициентами уравнение реакции 3 Mn (NO3)2 + 2 KMnO4 + 2 H2O → 4 HNO3 + 5 MnO2 + 2 KNO3 с дополненными продуктами. Приложение для вычисления и …
 Balance the reaction of HNO3 + KMnO4 = NO3 + KMn + H2O using this chemical equation balancer!
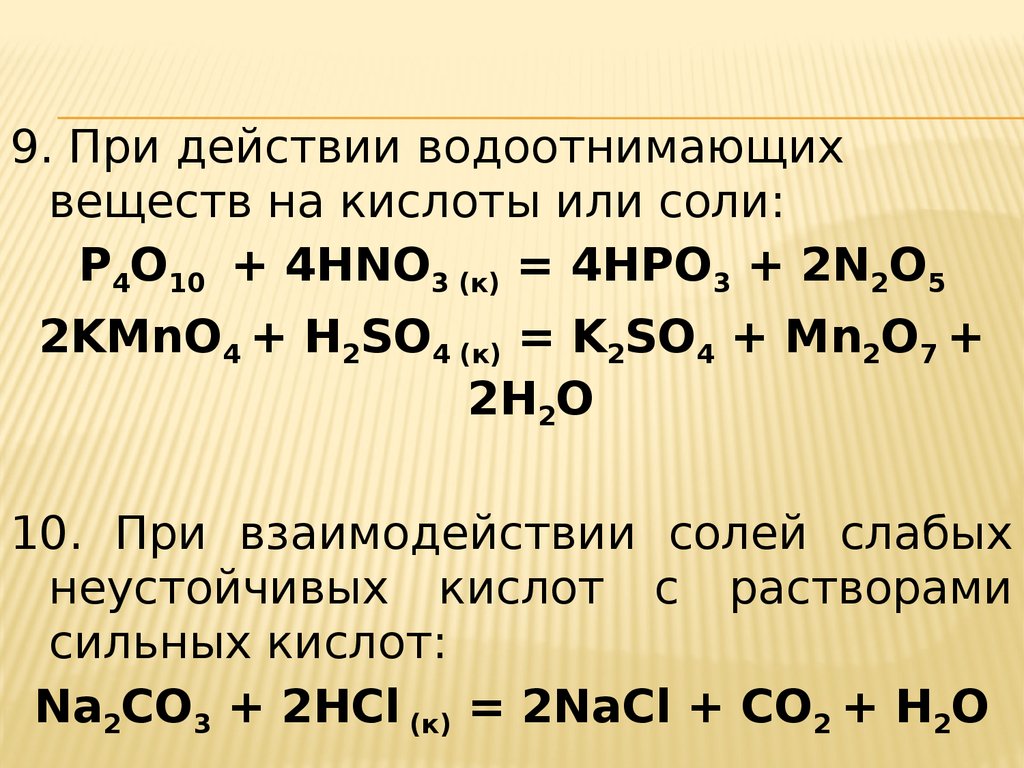
Balance the reaction of HNO3 + KMnO4 = NO3 + KMn + H2O using this chemical equation balancer!
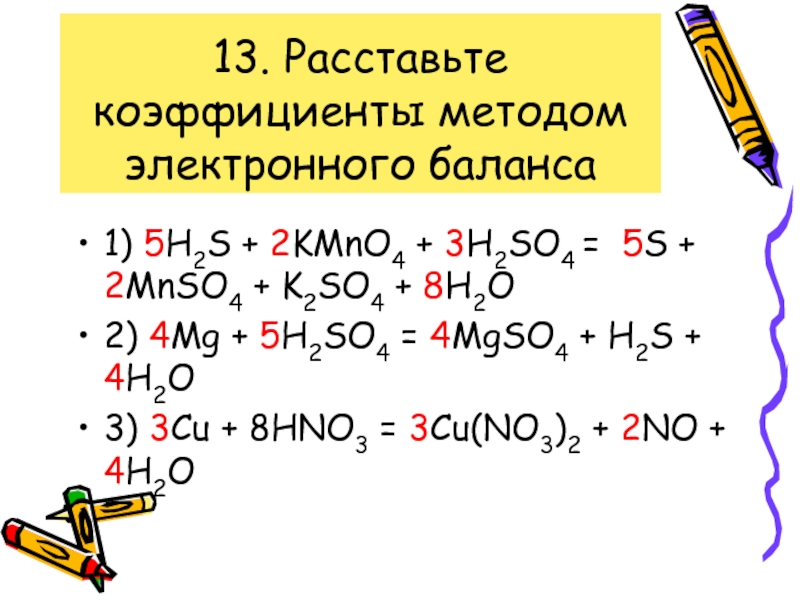
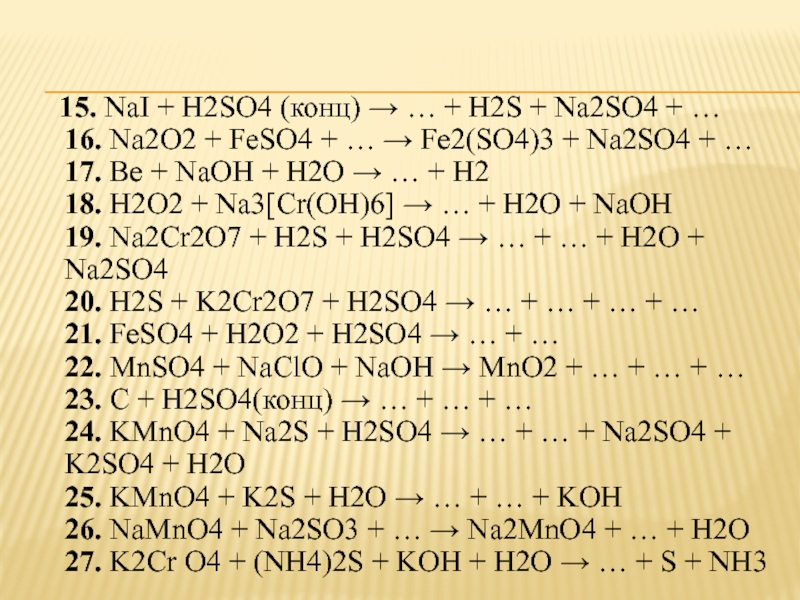
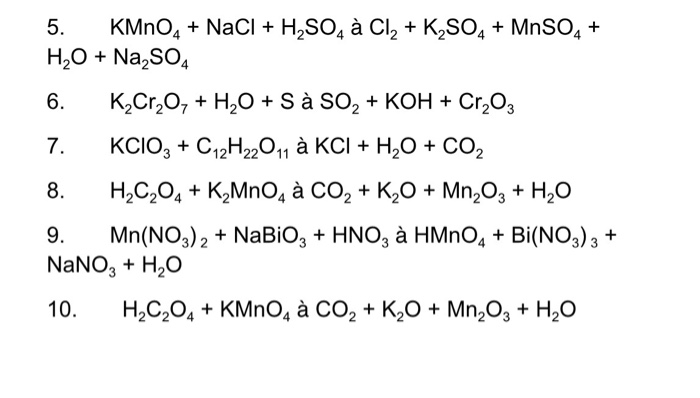
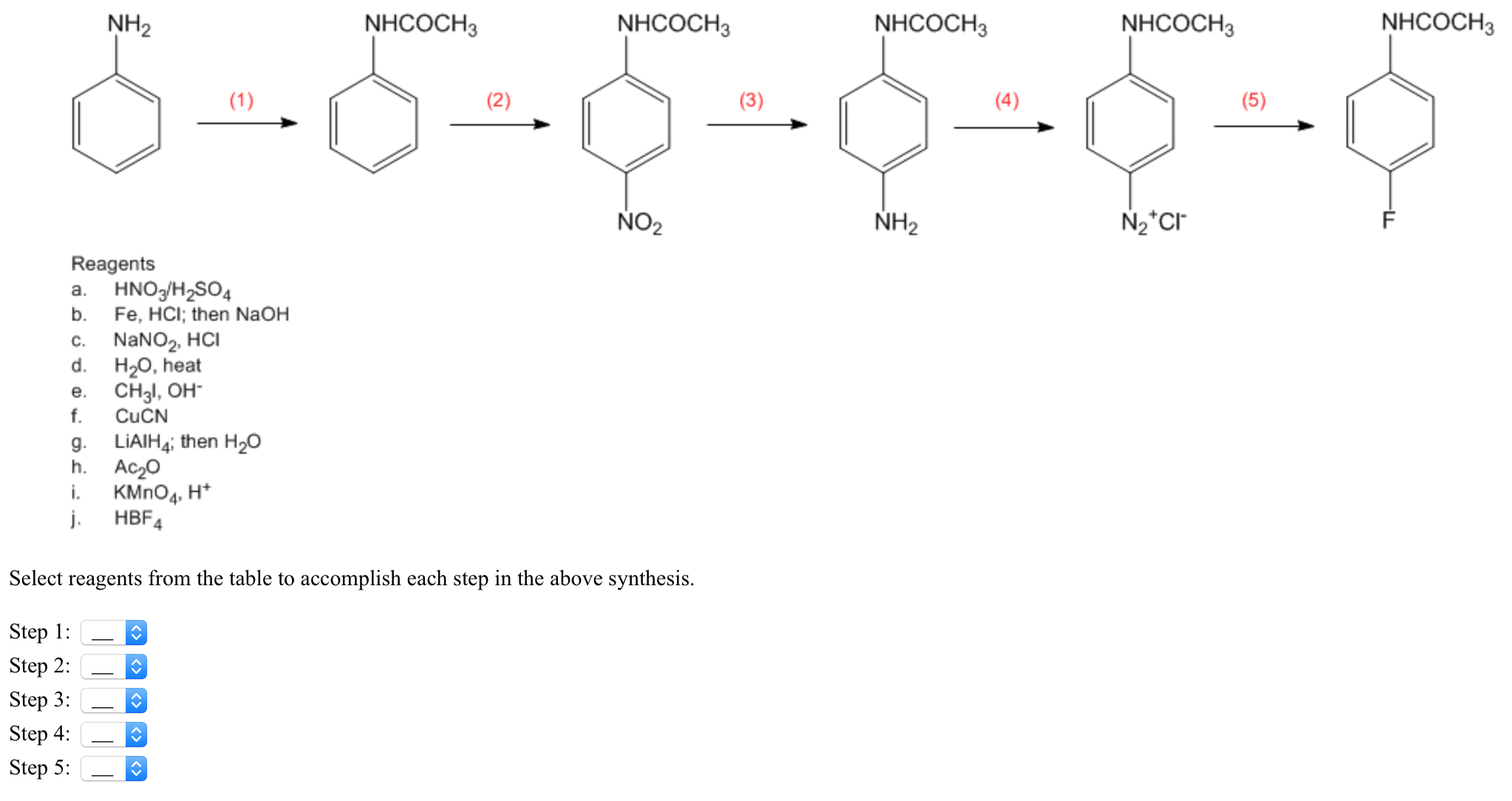
 Balance the following equations of redox reactions: Assign oxidation numbers to all elements in the reaction. Separate the redox reaction into two half reactions. Balance the atoms in each …
Balance the following equations of redox reactions: Assign oxidation numbers to all elements in the reaction. Separate the redox reaction into two half reactions. Balance the atoms in each …
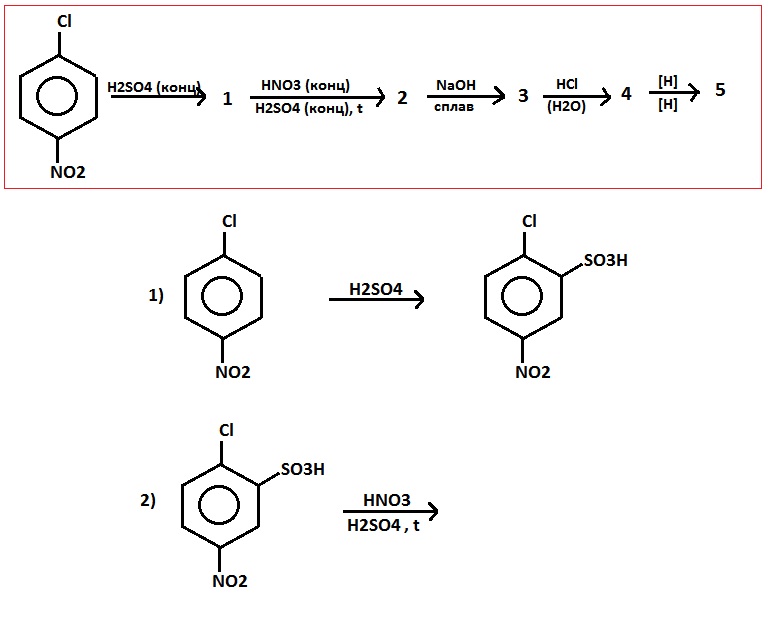
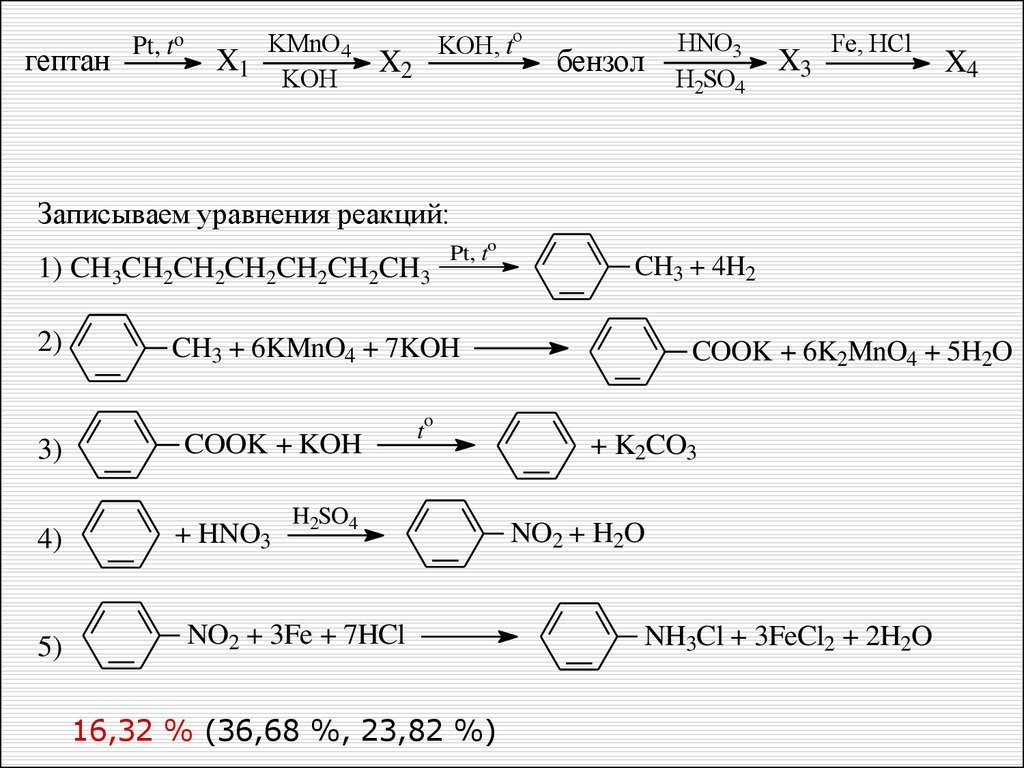
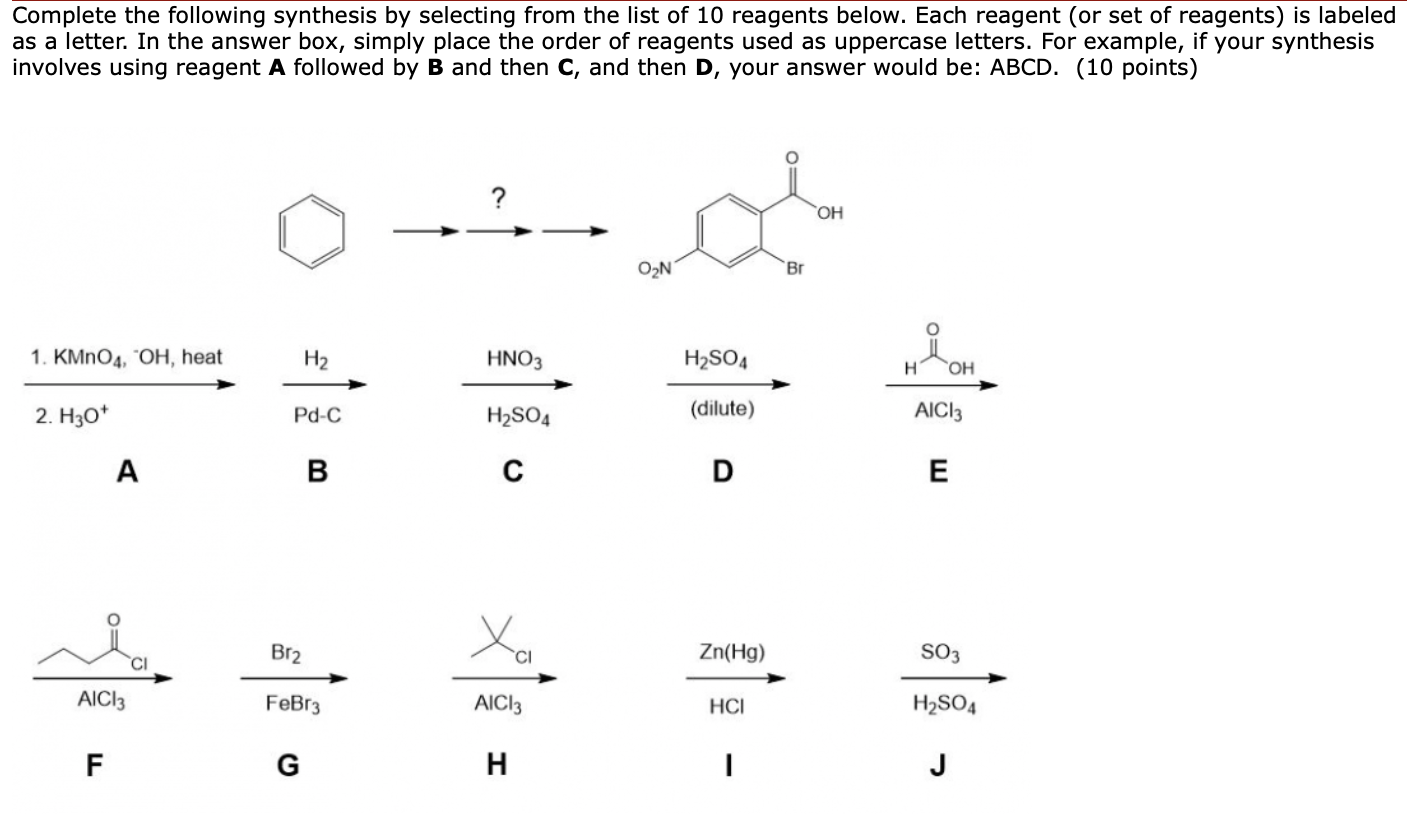
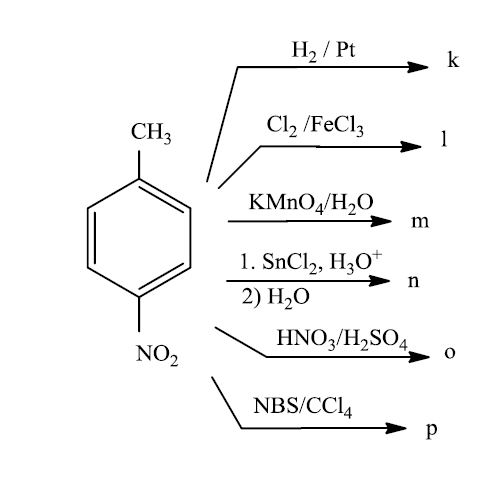
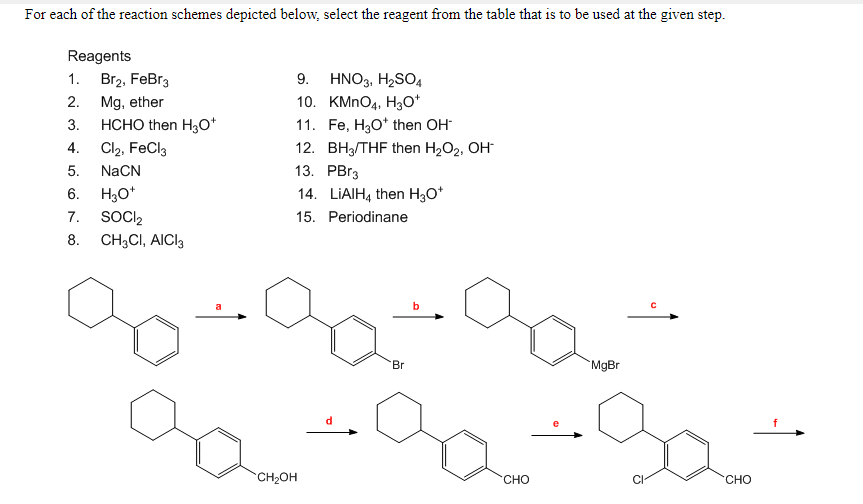
 Oxidation of aromatic alkanes with KMnO4 to give carboxylic acids. Description: Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. Notes: The position directly adjacent to an aromatic …
Oxidation of aromatic alkanes with KMnO4 to give carboxylic acids. Description: Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. Notes: The position directly adjacent to an aromatic …
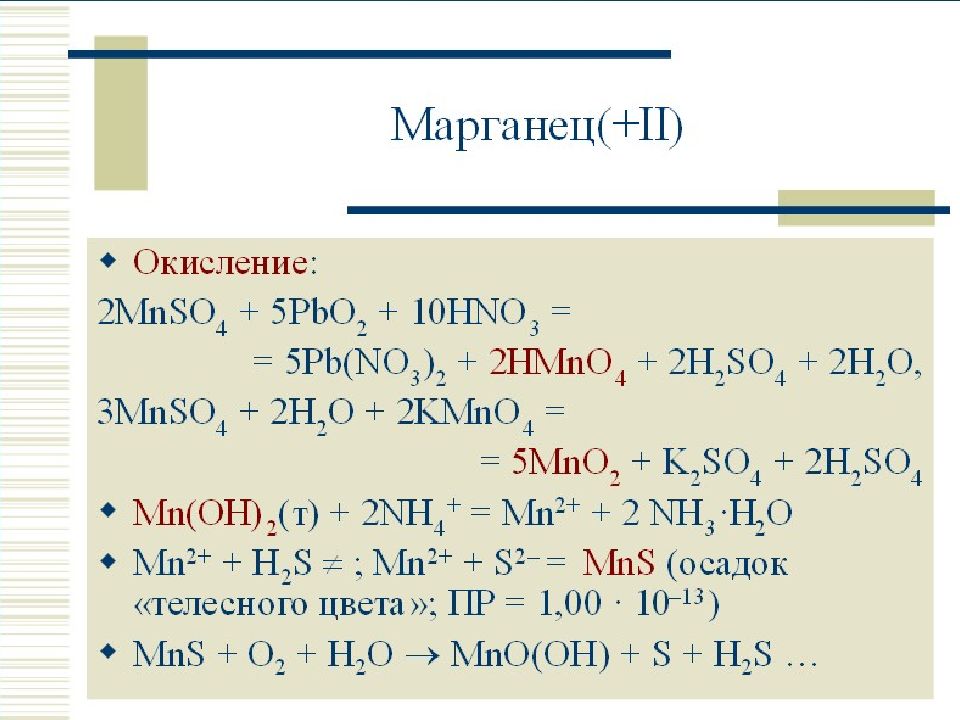 Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The limiting reagent row will be highlighted in pink. Examples of …
Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The limiting reagent row will be highlighted in pink. Examples of …
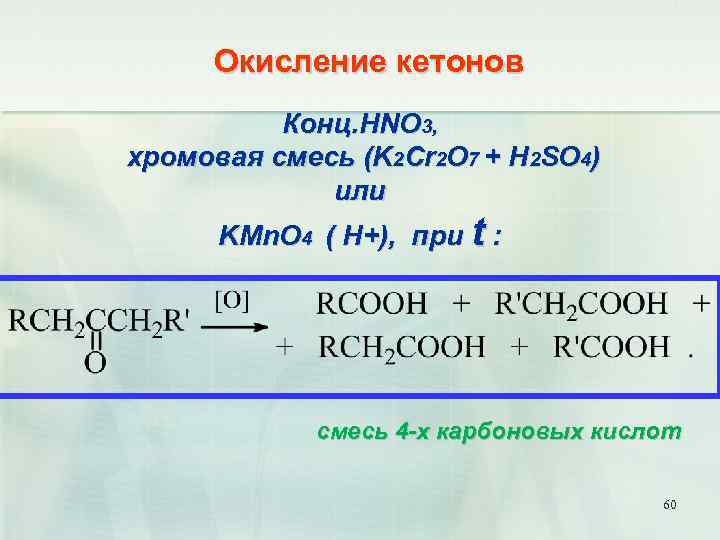 Balance the reaction of KMnO4 + NH3 = KNO3 + MnO2 + KOH + H2O using this chemical equation balancer!
Balance the reaction of KMnO4 + NH3 = KNO3 + MnO2 + KOH + H2O using this chemical equation balancer!
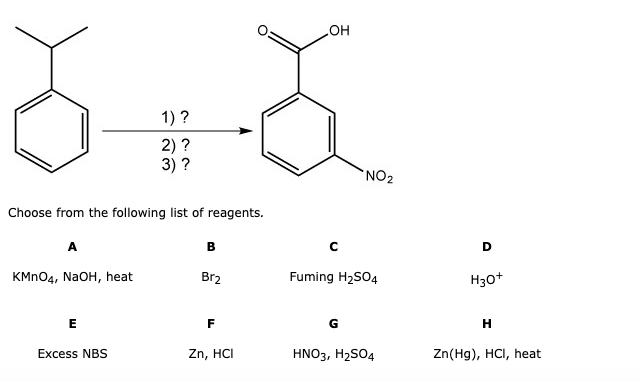 Balancing with algebraic method. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable (like x, y, z), and a series of equations are set up based on the number of each type of atom.
Balancing with algebraic method. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable (like x, y, z), and a series of equations are set up based on the number of each type of atom.
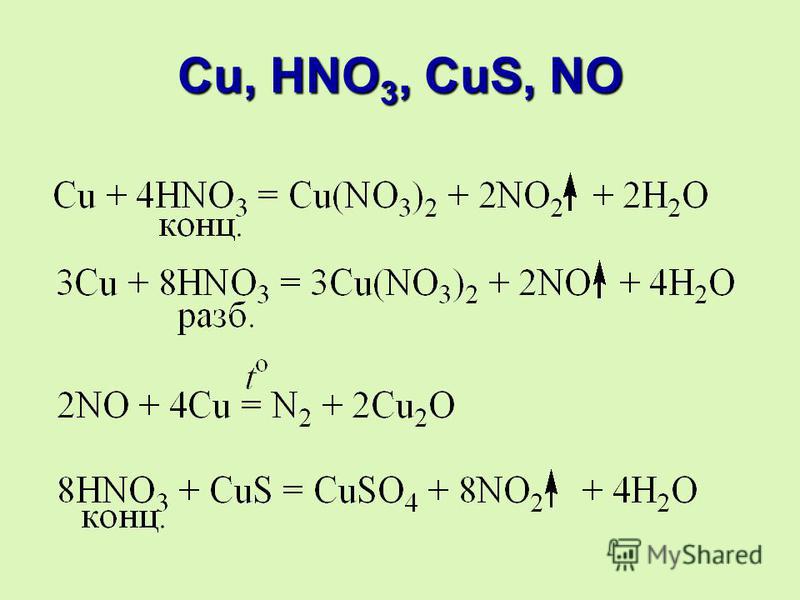
 I actually do not really expect any reaction between KMnO4 and 65% HNO3. Some KMnO4 may dissolve, giving a deep purple solution. Both are strong oxidizers and they do not …
I actually do not really expect any reaction between KMnO4 and 65% HNO3. Some KMnO4 may dissolve, giving a deep purple solution. Both are strong oxidizers and they do not …
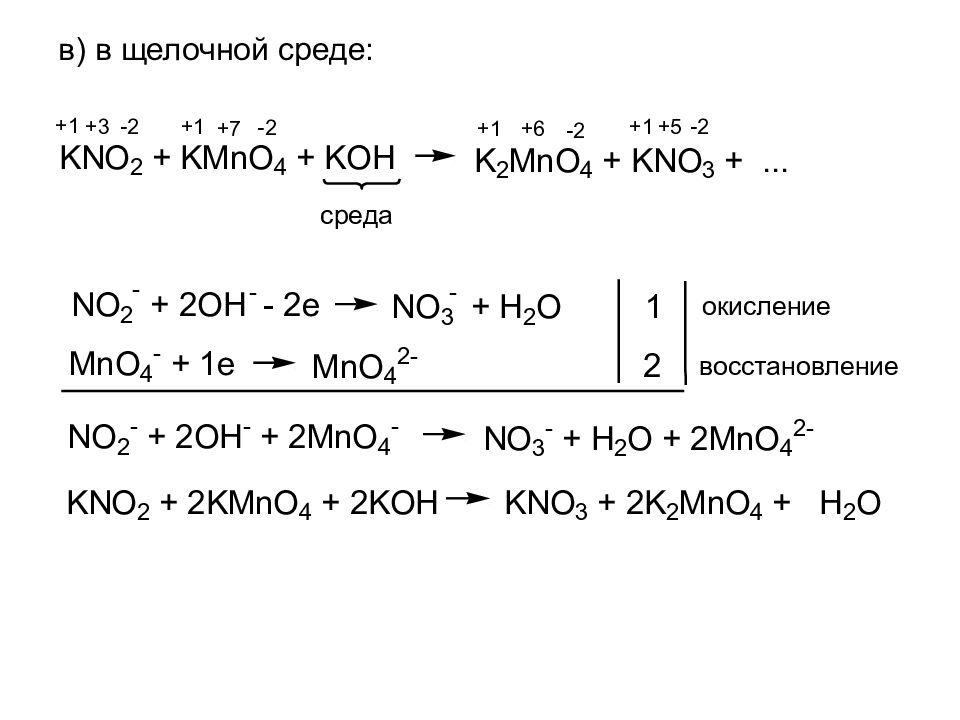
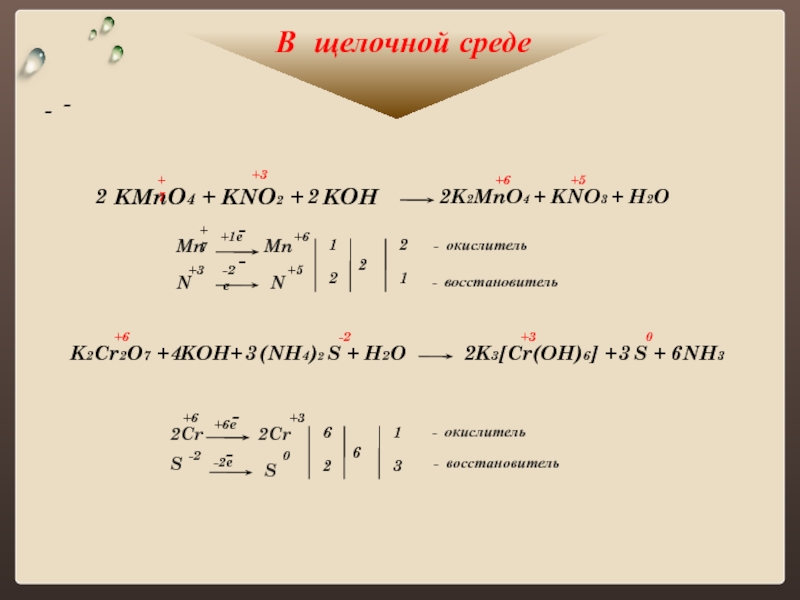
 KMnO4 + KNO2 + H2O = MnO2 + KOH + HNO3 - Сбалансированное химическое уравнение, лимитирующий реагент и стехиометрия. Балансировка химического уравнения - онлайн …
KMnO4 + KNO2 + H2O = MnO2 + KOH + HNO3 - Сбалансированное химическое уравнение, лимитирующий реагент и стехиометрия. Балансировка химического уравнения - онлайн …
 In this lecture, we will be discussing, Why Potassium Permanganate - KMnO4 is not acidified with HCl & HNO3?Highlights:For Acidification of KMnO4, HCl and HN.
In this lecture, we will be discussing, Why Potassium Permanganate - KMnO4 is not acidified with HCl & HNO3?Highlights:For Acidification of KMnO4, HCl and HN.
 What is the redox reaction of KMnO4 and HNO3 acid? Well, both permanganate, and nitric acid are POTENT oxidants… if some nitrous acid were added, we would get formation of …
What is the redox reaction of KMnO4 and HNO3 acid? Well, both permanganate, and nitric acid are POTENT oxidants… if some nitrous acid were added, we would get formation of …
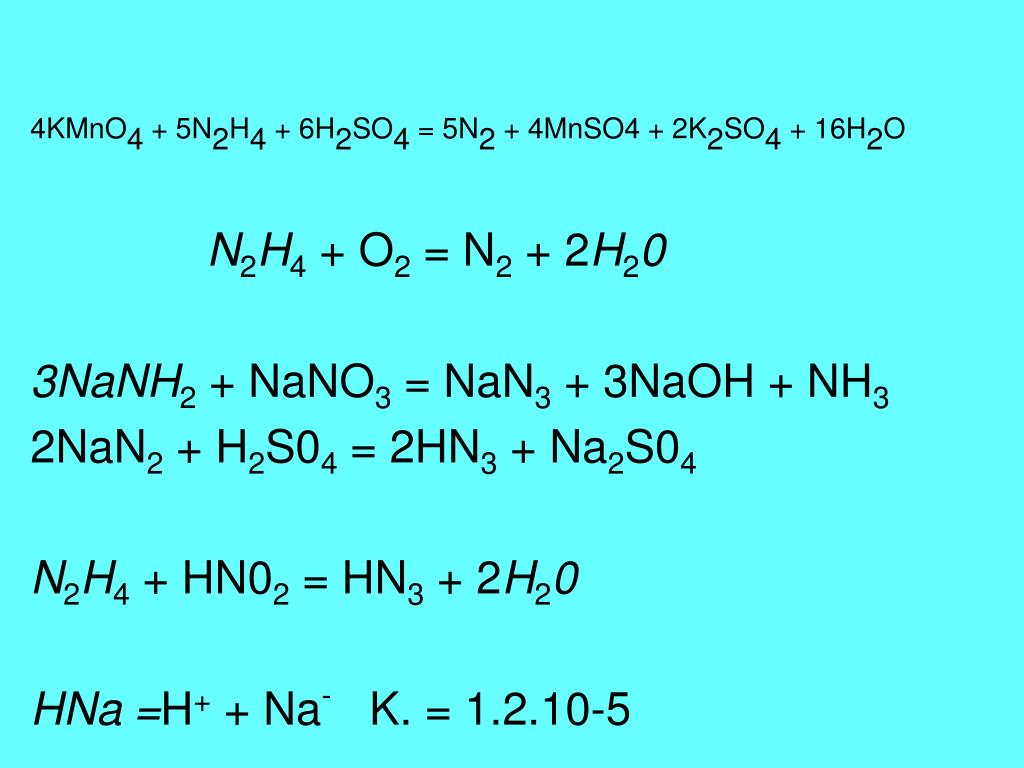
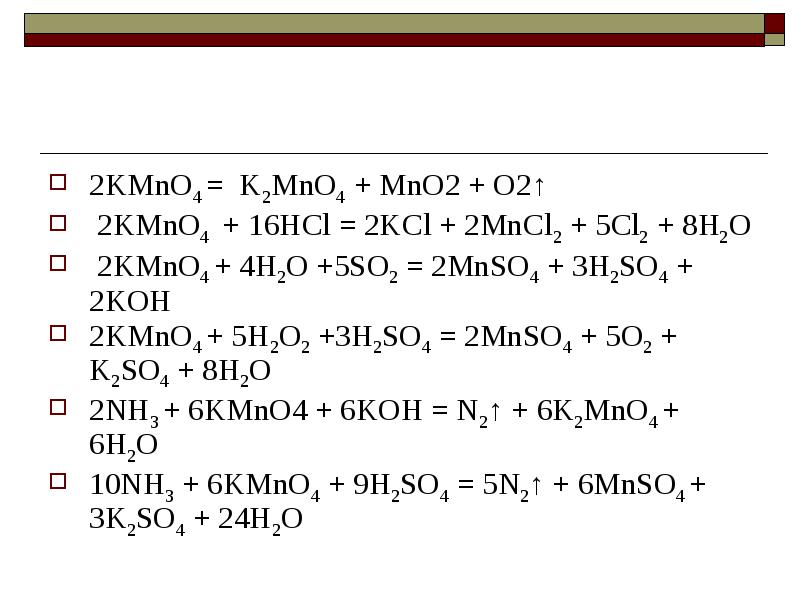
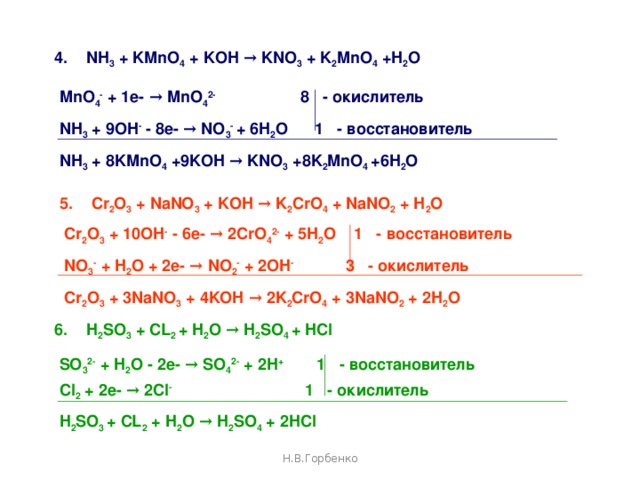
 KMnO4 является окислителем, HNO2 является восстановителем. Решенное и коэффициентами уравнение реакции 2 KMnO4 + 5 HNO2 + 3 H2SO4 → 2 MnSO4 + …
KMnO4 является окислителем, HNO2 является восстановителем. Решенное и коэффициентами уравнение реакции 2 KMnO4 + 5 HNO2 + 3 H2SO4 → 2 MnSO4 + …
 Use 1 teaspoon of sugar, half a teaspoon of NaOH and add half a teaspoon of KMnO4, dissolved in water. MnO2 does not form Mn (NO3)2 with HNO3 though. You also …
Use 1 teaspoon of sugar, half a teaspoon of NaOH and add half a teaspoon of KMnO4, dissolved in water. MnO2 does not form Mn (NO3)2 with HNO3 though. You also …
Еще по теме:



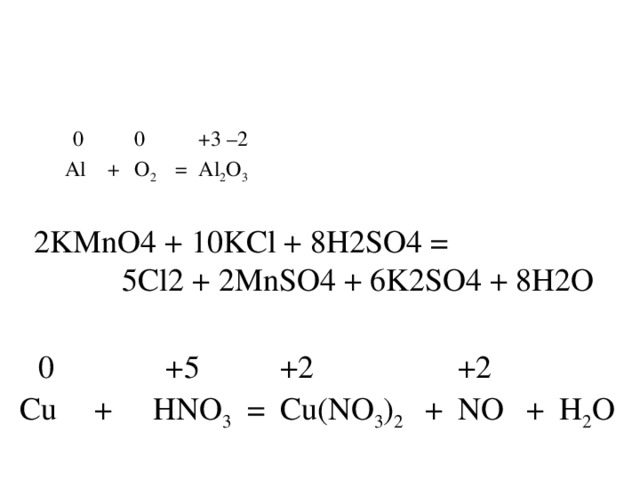
Еще по теме: