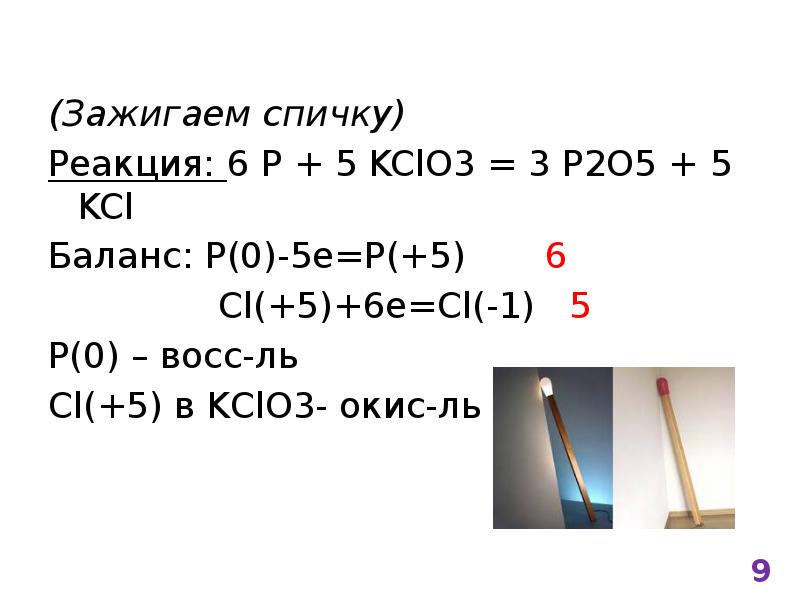
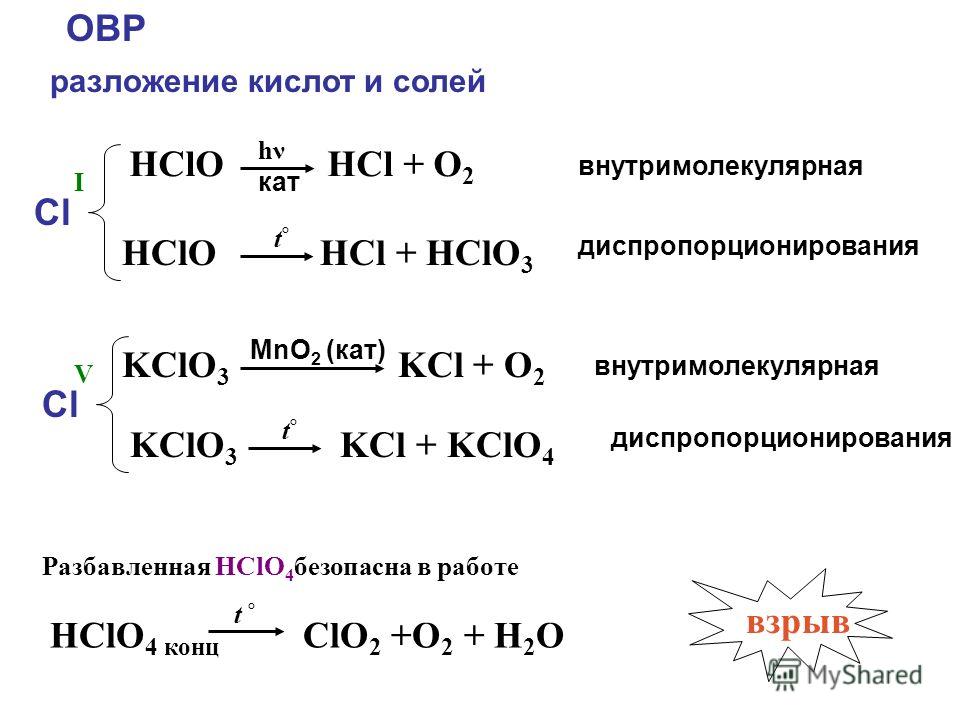
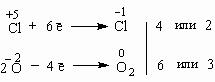2KClO3 → 2KCl + 3O2. Реакция термического разложения хлората калия. В результате реакции образуются хлорид калия и кислород. Реакция протекает при …
2KClO3 → 2KCl + 3O2. Реакция термического разложения хлората калия. В результате реакции образуются хлорид калия и кислород. Реакция протекает при …
 2KClO3 ---> 2KCl + 3O2 - Balanced chemical equation, limiting reagent and stoichiometry. Balance Chemical Equation - Online Balancer. Enter a chemical equation to balance: …
2KClO3 ---> 2KCl + 3O2 - Balanced chemical equation, limiting reagent and stoichiometry. Balance Chemical Equation - Online Balancer. Enter a chemical equation to balance: …
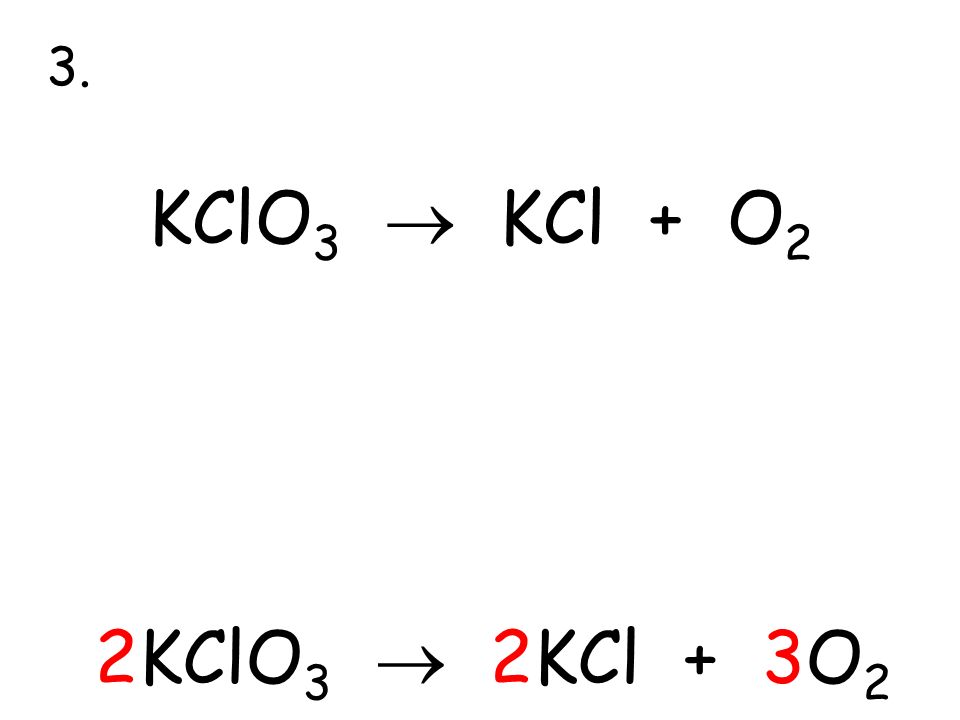 Решенное и коэффициентами уравнение реакции 2 KClO3 → 2 KCl + 3 O2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 KClO3 → 2 KCl + 3 O2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
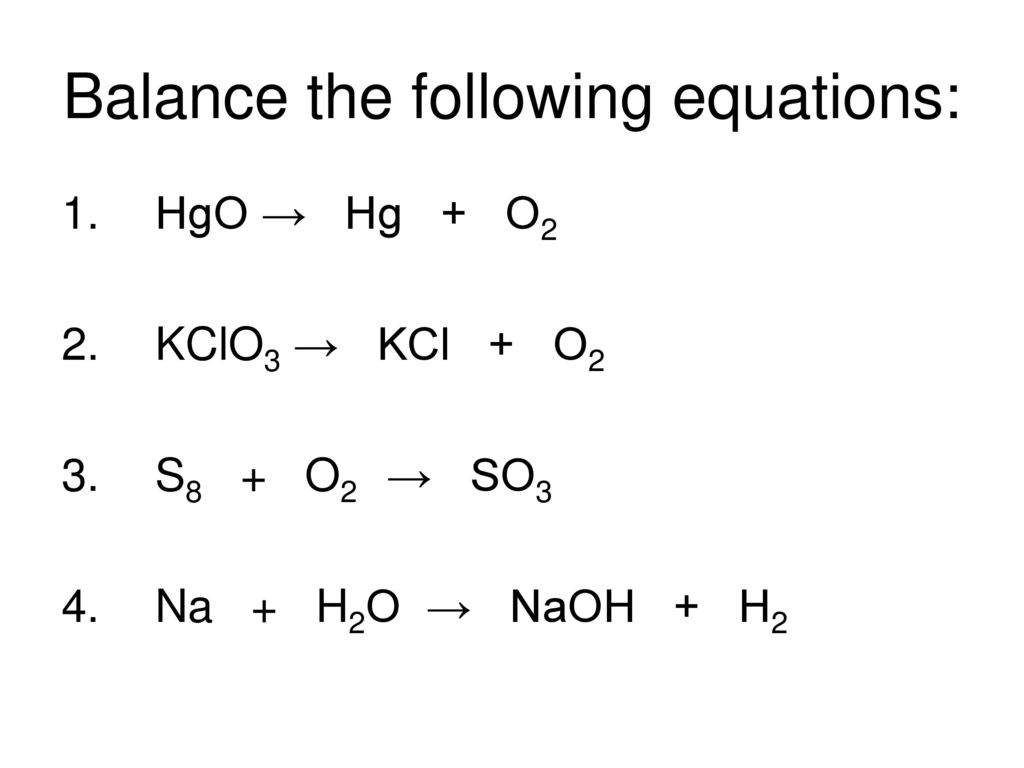
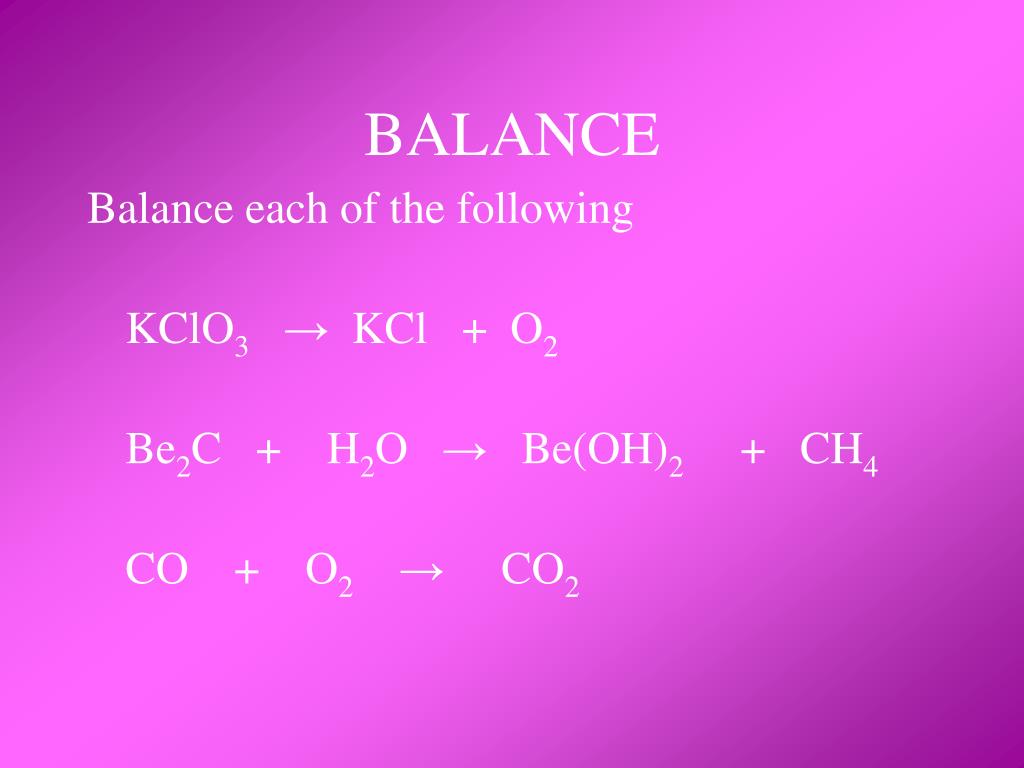
 To balance a chemical equation: 1. Every element must have the same number of atoms on each side of the equation (law of conservation of mass). 2. Balancing the equation can be done only …
To balance a chemical equation: 1. Every element must have the same number of atoms on each side of the equation (law of conservation of mass). 2. Balancing the equation can be done only …
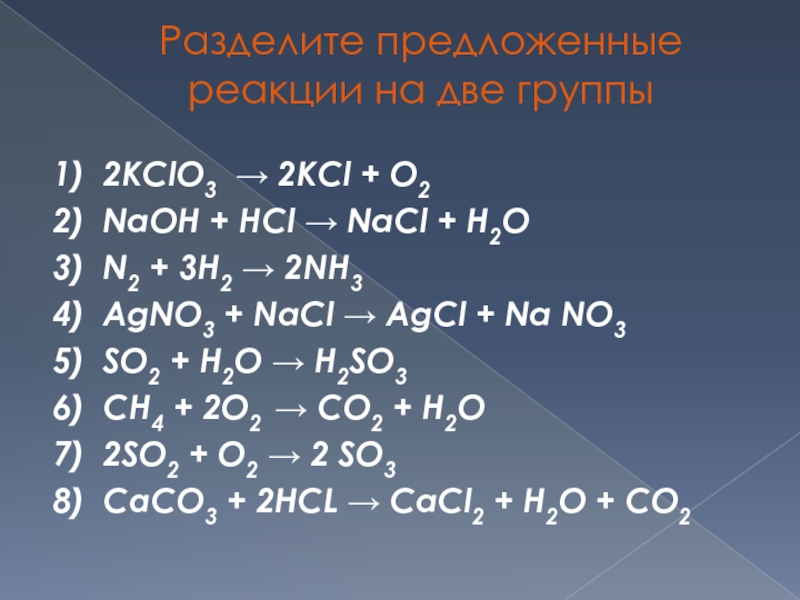
 2kclo3-->2kcl+3o2 ( проверив мы должны проверить число атомов (индексы) и количество молекул)
2kclo3-->2kcl+3o2 ( проверив мы должны проверить число атомов (индексы) и количество молекул)
 2 K Cl O 3 (aq) → 2 K Cl (aq) + 3 O 2 (g) This is an oxidation-reduction (redox) reaction: KClO3 is an oxidizing agent, KClO3 is a reducing agent. This is a gas evolution reaction, O2 is the …
2 K Cl O 3 (aq) → 2 K Cl (aq) + 3 O 2 (g) This is an oxidation-reduction (redox) reaction: KClO3 is an oxidizing agent, KClO3 is a reducing agent. This is a gas evolution reaction, O2 is the …
 Learn why potassium chlorate (KClO3) decomposes into potassium chloride (KCl) and oxygen gas (O2) when heated. See the reaction equation, the stability of the products, and an example of fireworks.
Learn why potassium chlorate (KClO3) decomposes into potassium chloride (KCl) and oxygen gas (O2) when heated. See the reaction equation, the stability of the products, and an example of fireworks.
 $$\ce{energy + 2KClO3 (s) <=> 2KCl(s) + 3O2 (g)}$$ The reaction will be more active in the reverse direction if: $\ce{O_2}$ is added ; more $\ce{KCl}$ salt is added. More $\ce{KClO3}$ …
$$\ce{energy + 2KClO3 (s) <=> 2KCl(s) + 3O2 (g)}$$ The reaction will be more active in the reverse direction if: $\ce{O_2}$ is added ; more $\ce{KCl}$ salt is added. More $\ce{KClO3}$ …
 The given reaction is: 2 KClO 3 (s) Potassium chlorate → 2 KCl (s) Potassium chloride + 3 O 2 (g) Oxygen.
The given reaction is: 2 KClO 3 (s) Potassium chlorate → 2 KCl (s) Potassium chloride + 3 O 2 (g) Oxygen.
 Free Pre-Algebra, Algebra, Trigonometry, Calculus, Geometry, Statistics and Chemistry calculators step-by-step.
Free Pre-Algebra, Algebra, Trigonometry, Calculus, Geometry, Statistics and Chemistry calculators step-by-step.
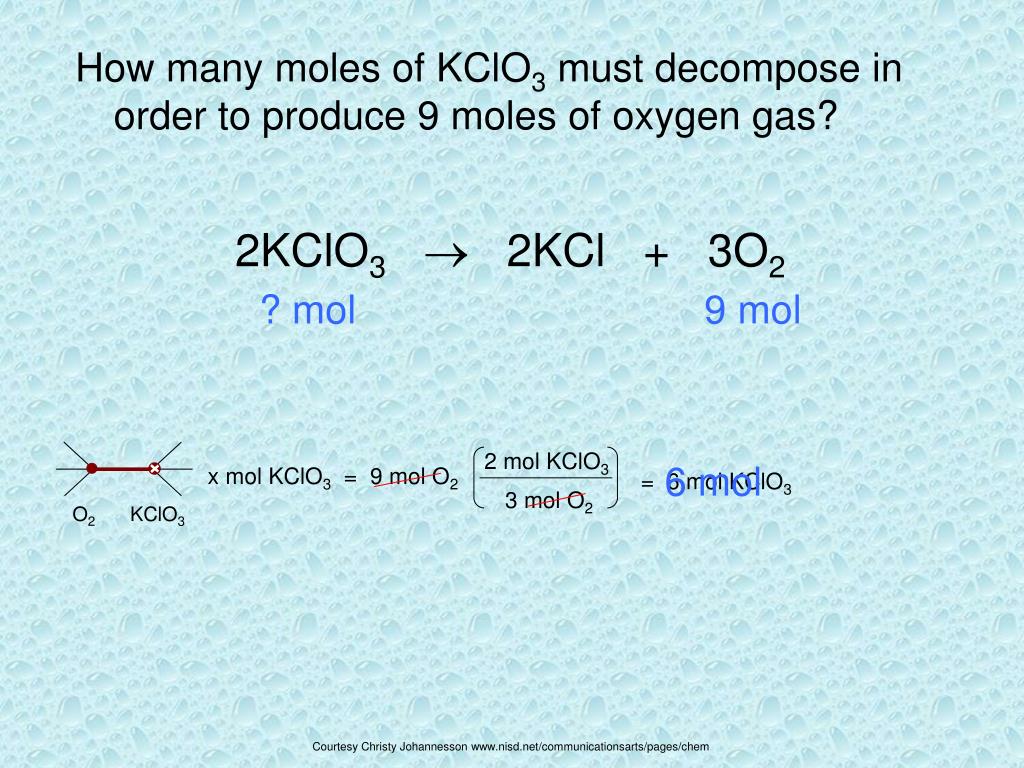
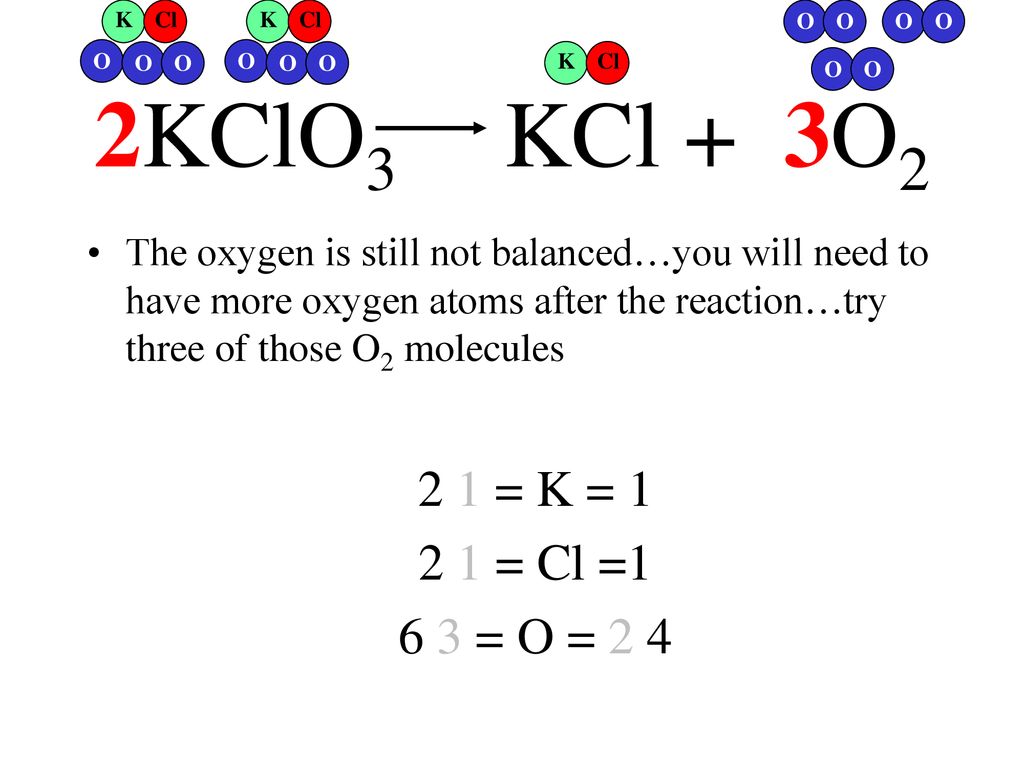
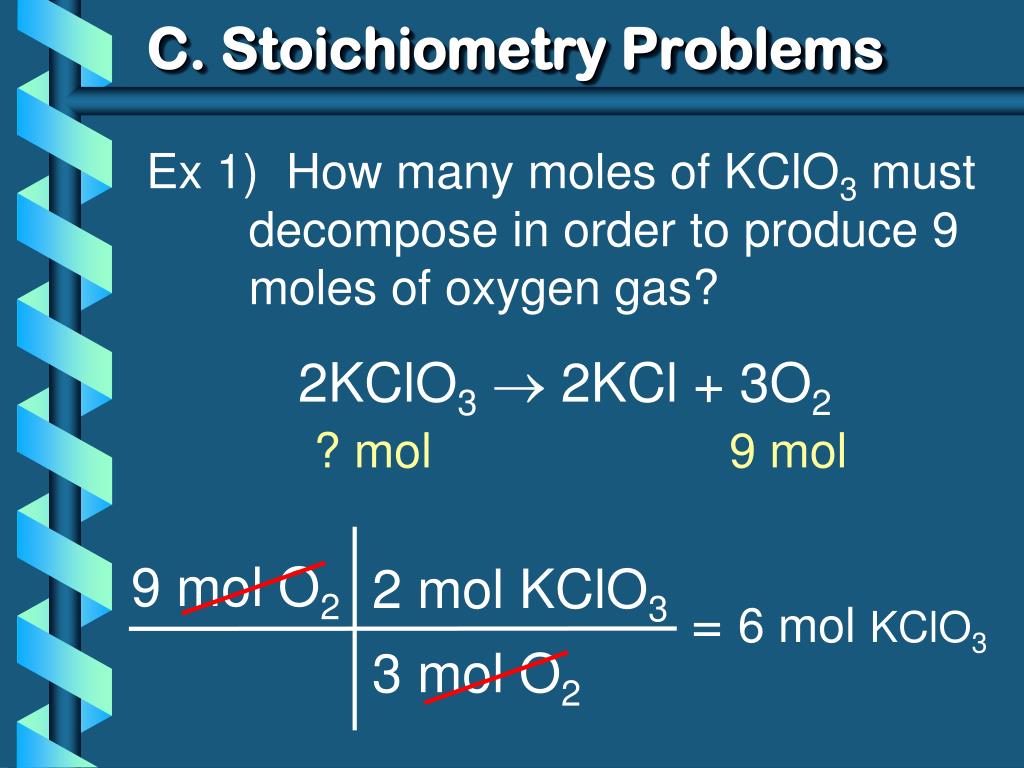
 2KClO 3 ==> 2KCl + 3O 2 This is the correctly balanced equation. If you look at it, it tells you that TWO moles of KClO 3 decompose to produce TWO moles of KCl and 3 …
2KClO 3 ==> 2KCl + 3O 2 This is the correctly balanced equation. If you look at it, it tells you that TWO moles of KClO 3 decompose to produce TWO moles of KCl and 3 …
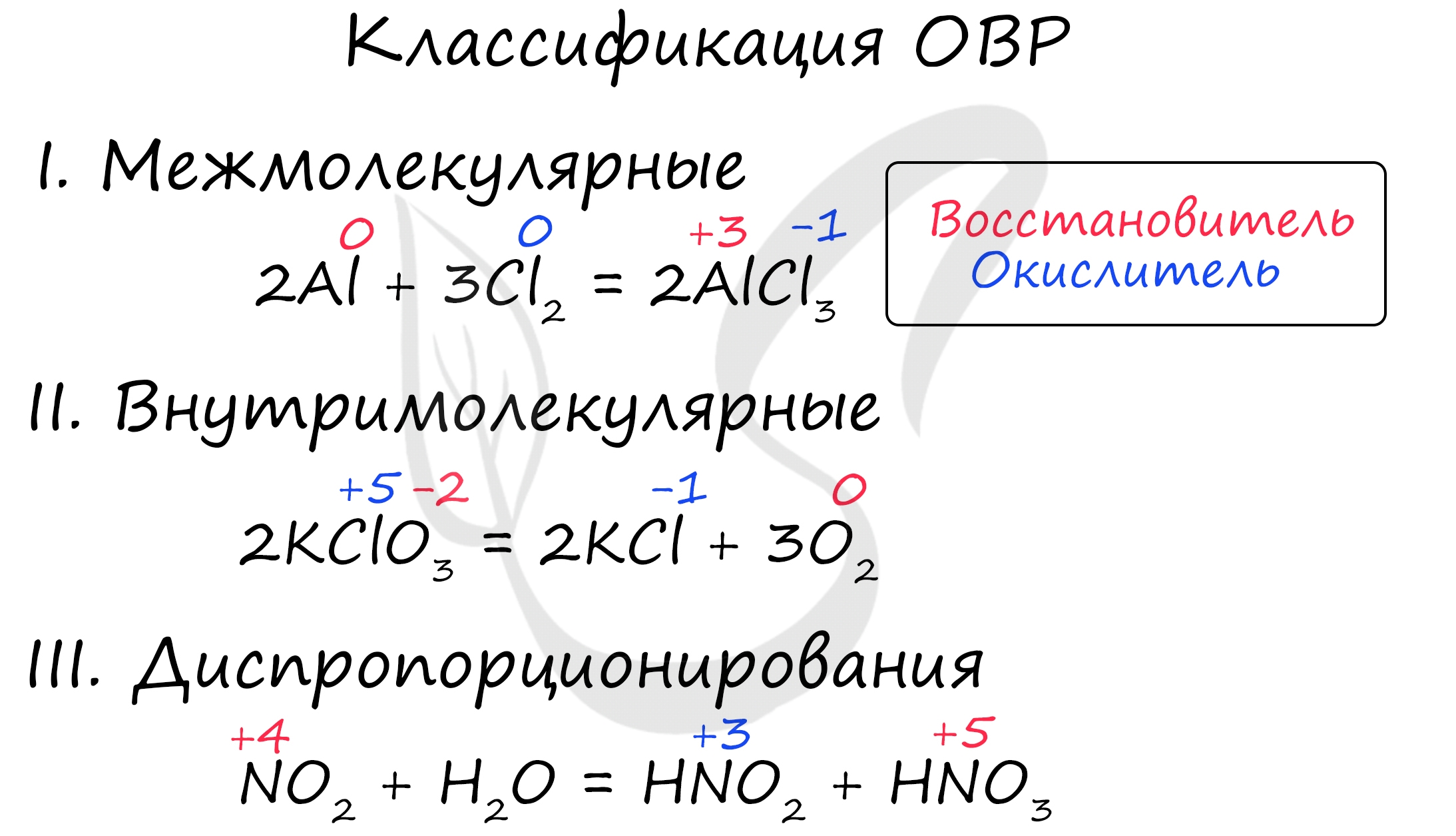 The problem stated is the decomposition of potassium chlorate into potassium chloride and oxygen gas, so. 2KClOX3 2KCl + 3OX2 ↑. Obviously, the half-reactions of this …
The problem stated is the decomposition of potassium chlorate into potassium chloride and oxygen gas, so. 2KClOX3 2KCl + 3OX2 ↑. Obviously, the half-reactions of this …
 2KClO 3 = 2KCl + 3O 2 - OBP, выделяется кислород, хлорид калия; Вычисления: M (KClO 3) = 122,6 г/моль; M (KCl) = 74,6г/моль; Y (KClO 3) = m / M = 12,25 / 122,6 = 0,09 моль; Y …
2KClO 3 = 2KCl + 3O 2 - OBP, выделяется кислород, хлорид калия; Вычисления: M (KClO 3) = 122,6 г/моль; M (KCl) = 74,6г/моль; Y (KClO 3) = m / M = 12,25 / 122,6 = 0,09 моль; Y …
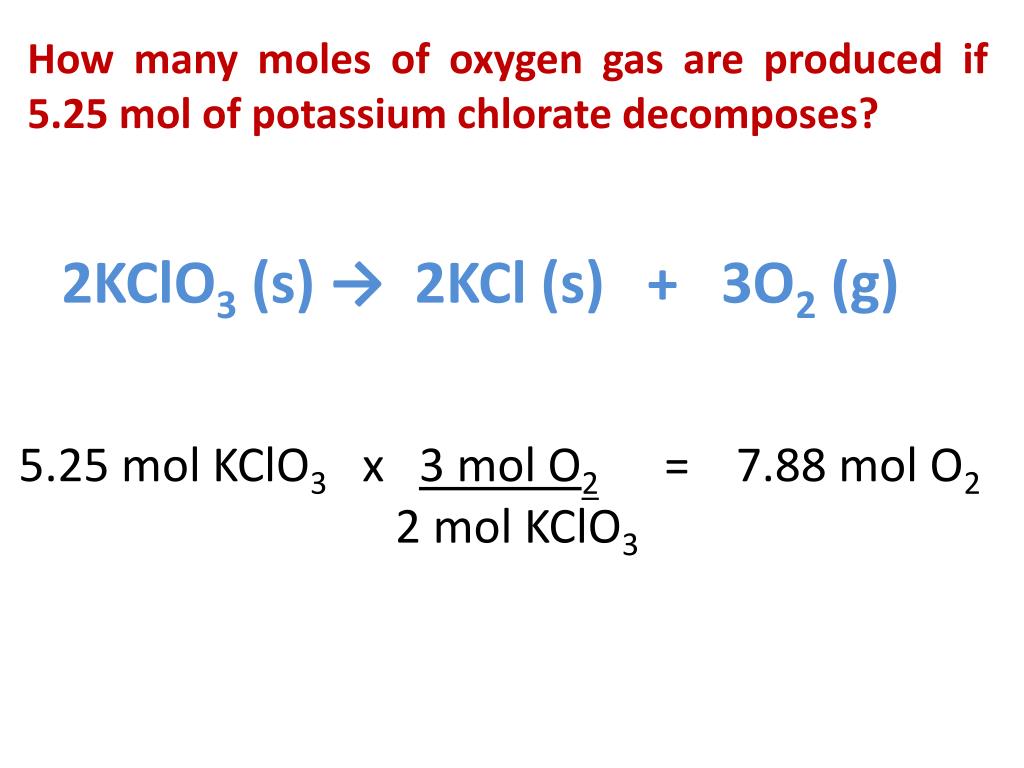
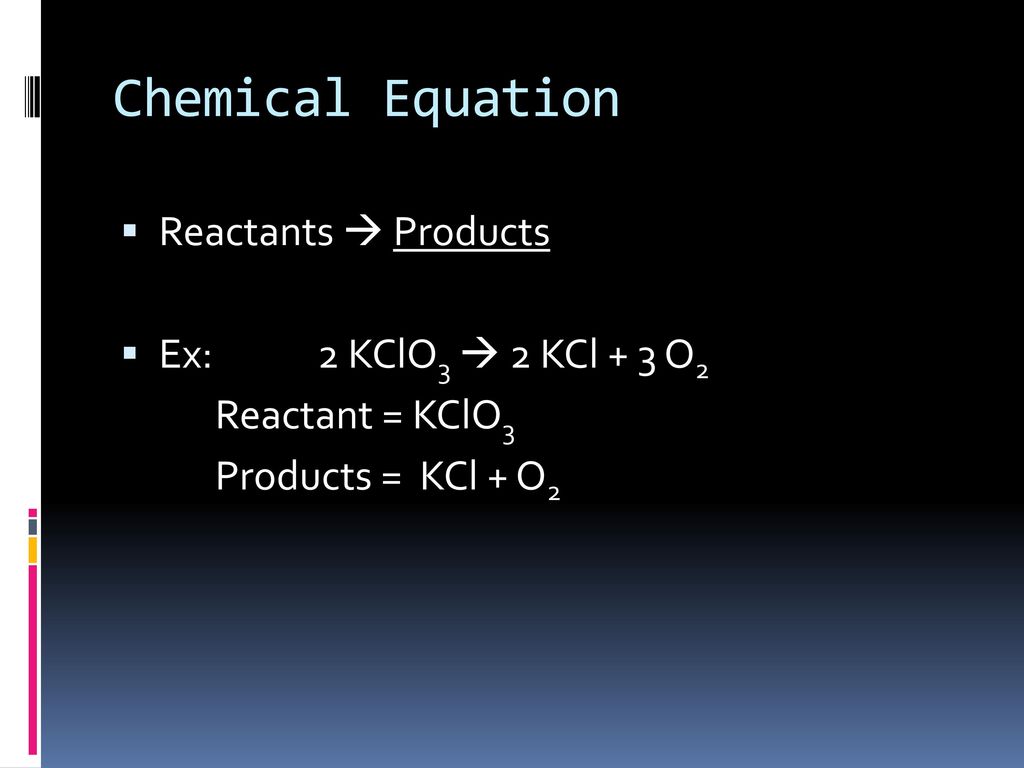
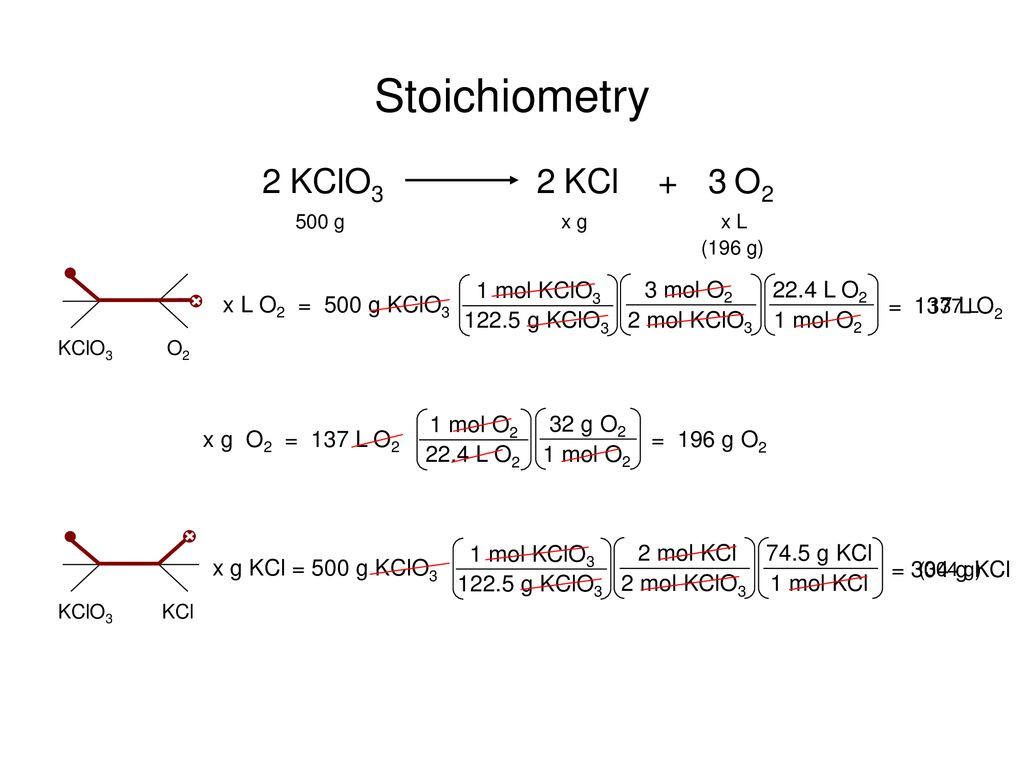
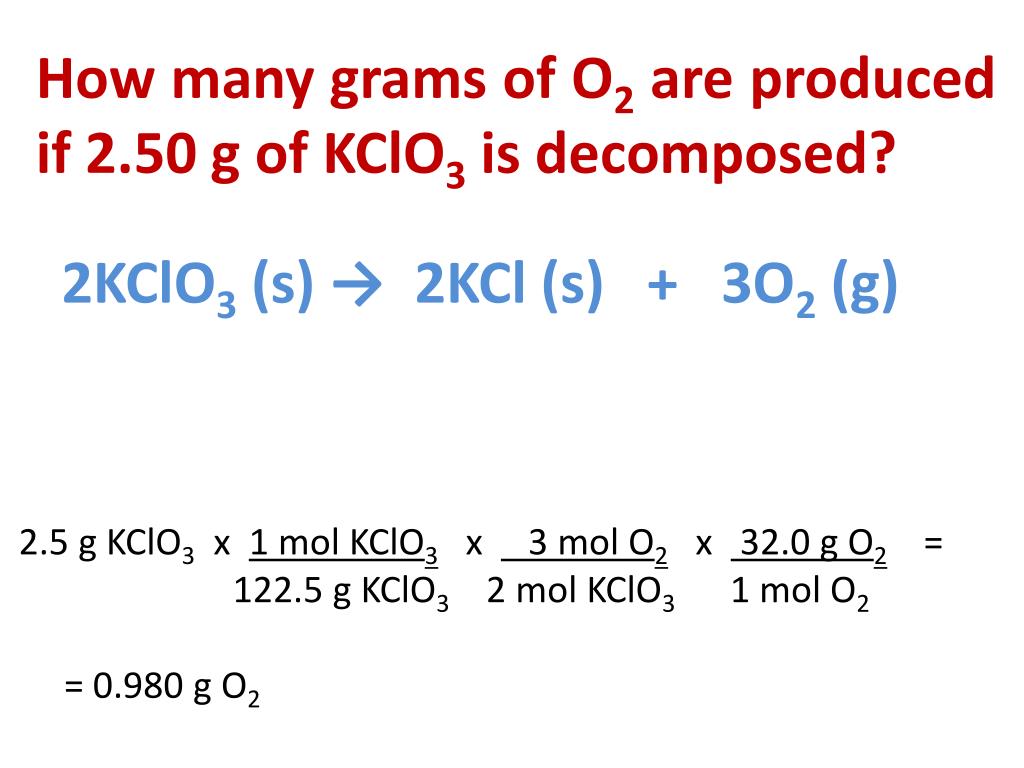
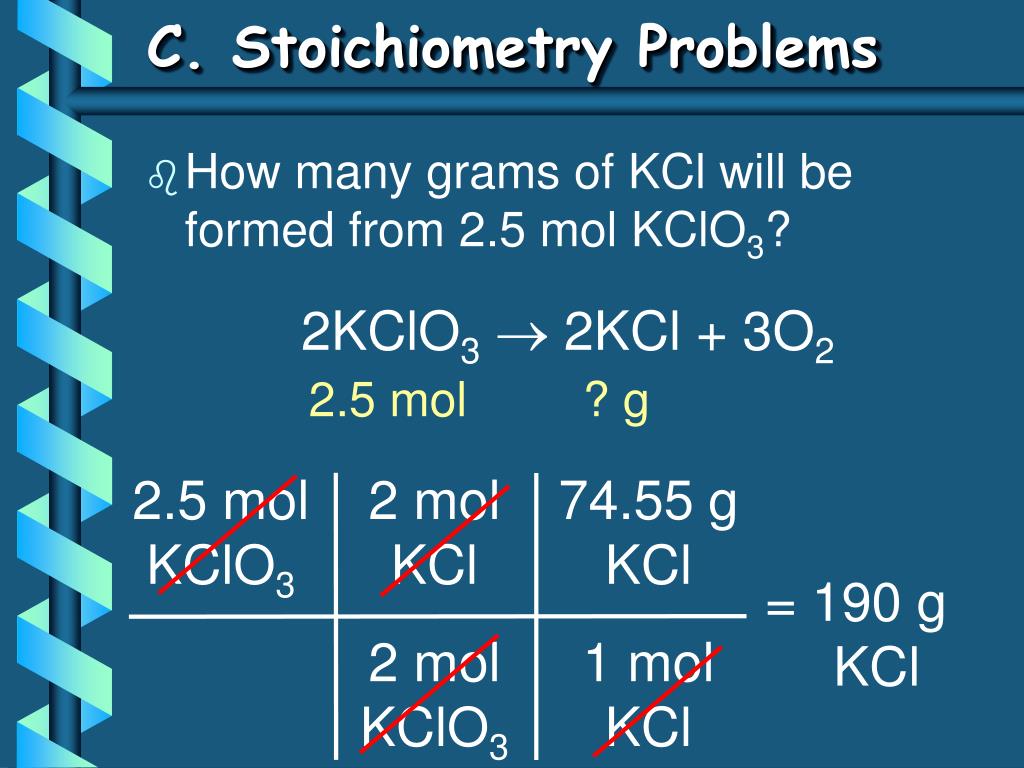
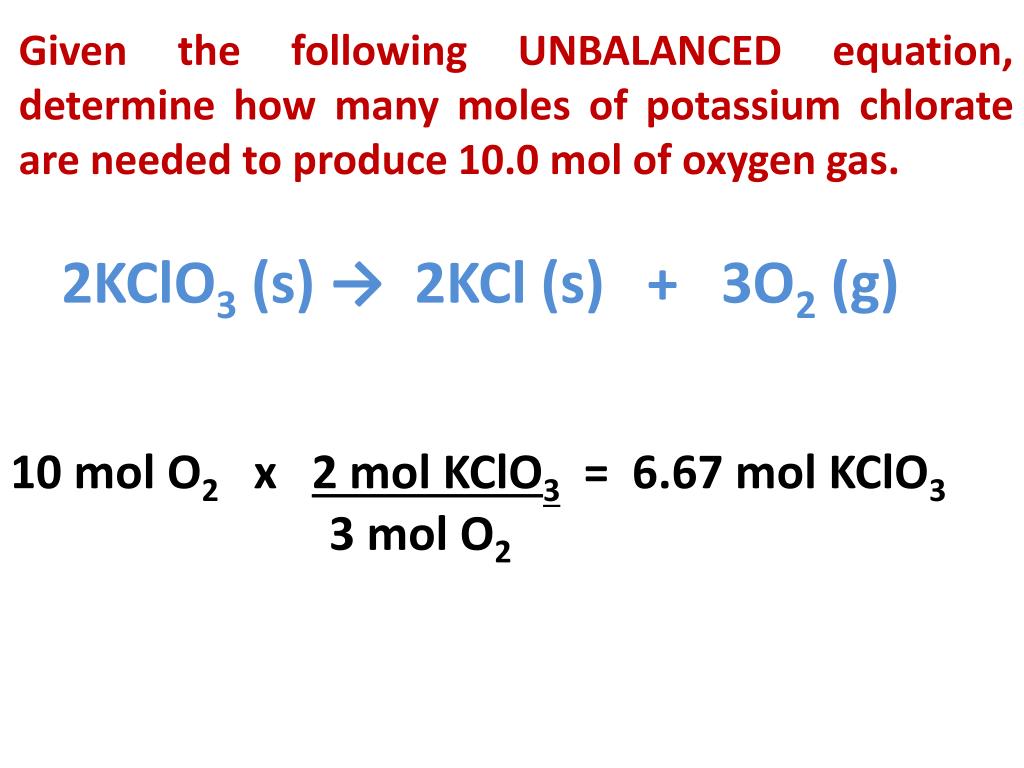
 Using the balanced equation, answer the following questions: 2KClO3 ---> 2KCl + 3O2. How many molecules of O2 will be formed from 5.0 grams of KClO3? (6 points) How many grams of …
Using the balanced equation, answer the following questions: 2KClO3 ---> 2KCl + 3O2. How many molecules of O2 will be formed from 5.0 grams of KClO3? (6 points) How many grams of …
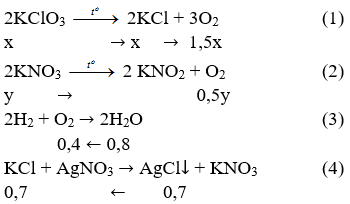
Еще по теме:






Еще по теме: