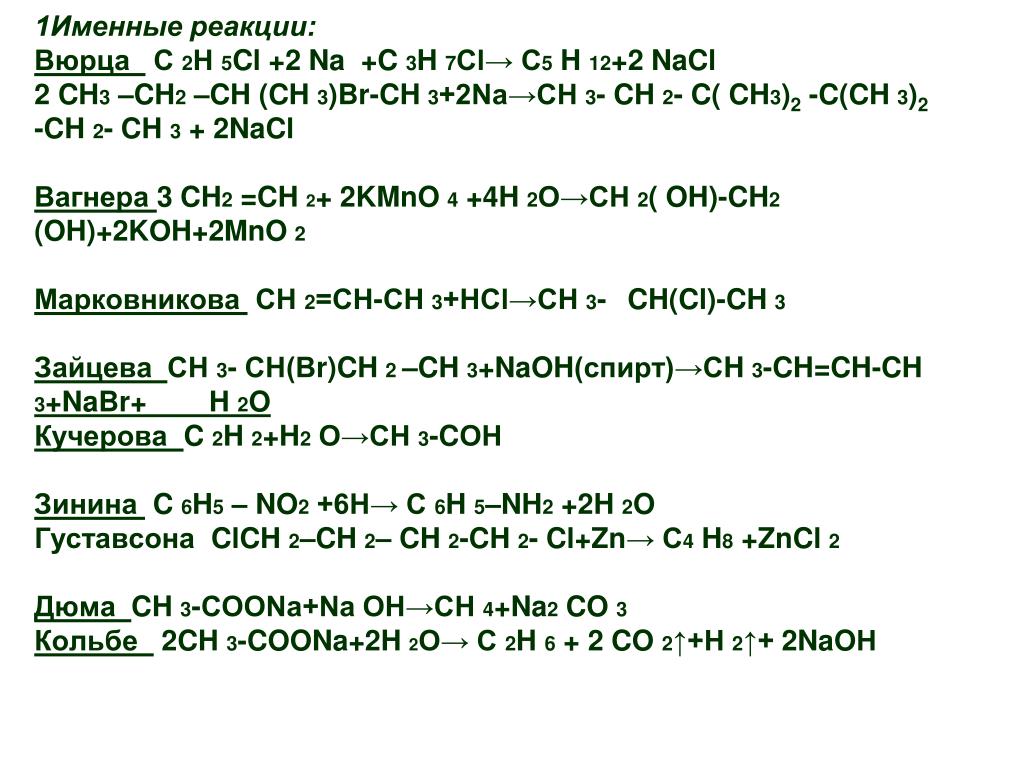
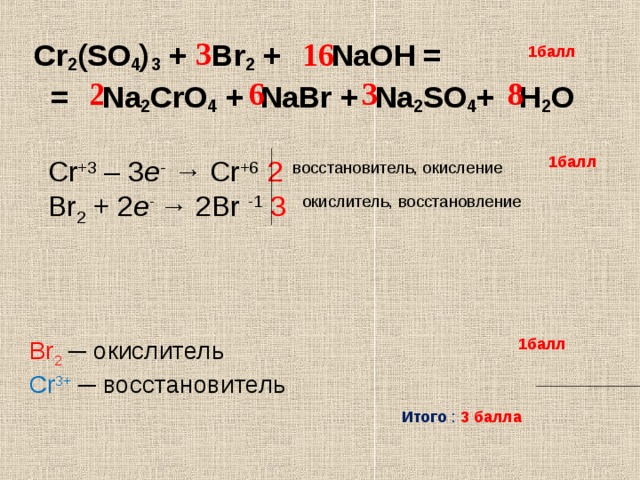
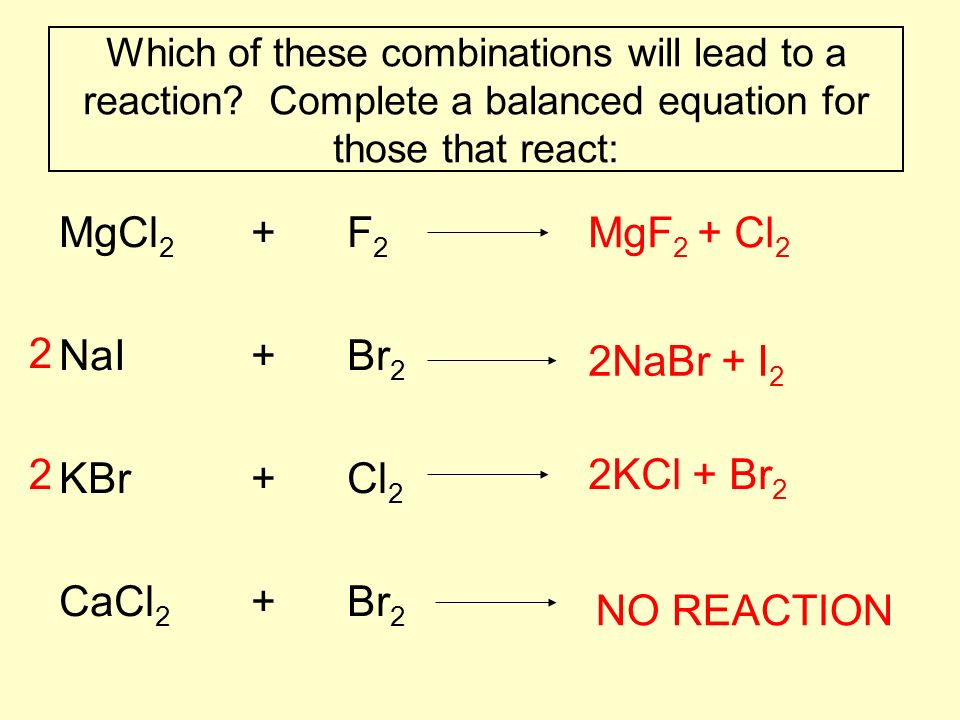
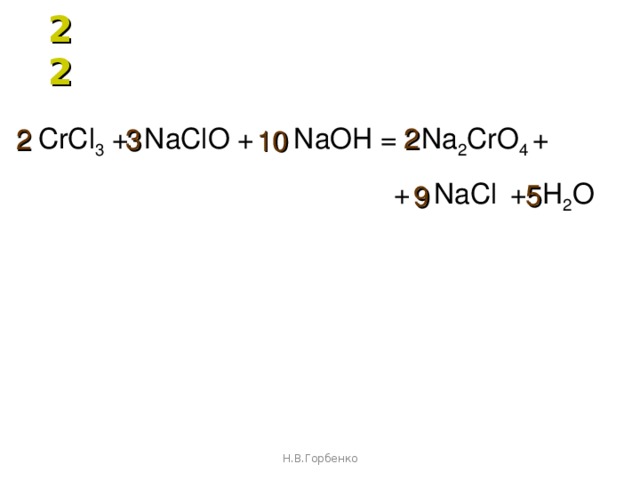Эта реакция в нормальных условиях не происходит, потому что Cl2 является более реакционноспособным чем Br2. Реакция должна быть обратной: NaCl + Br2 → Ø - Реакция не происходит Приложение для вычисления и дополнения продуктов реакции.
Эта реакция в нормальных условиях не происходит, потому что Cl2 является более реакционноспособным чем Br2. Реакция должна быть обратной: NaCl + Br2 → Ø - Реакция не происходит Приложение для вычисления и дополнения продуктов реакции.
 NaCl + Br2 = Cl2 + NaBr is a Single Displacement (Substitution) reaction where two moles of aqueous Sodium Chloride [NaCl] and one mole of liquid Dibromine [Br 2] react to form one …
NaCl + Br2 = Cl2 + NaBr is a Single Displacement (Substitution) reaction where two moles of aqueous Sodium Chloride [NaCl] and one mole of liquid Dibromine [Br 2] react to form one …
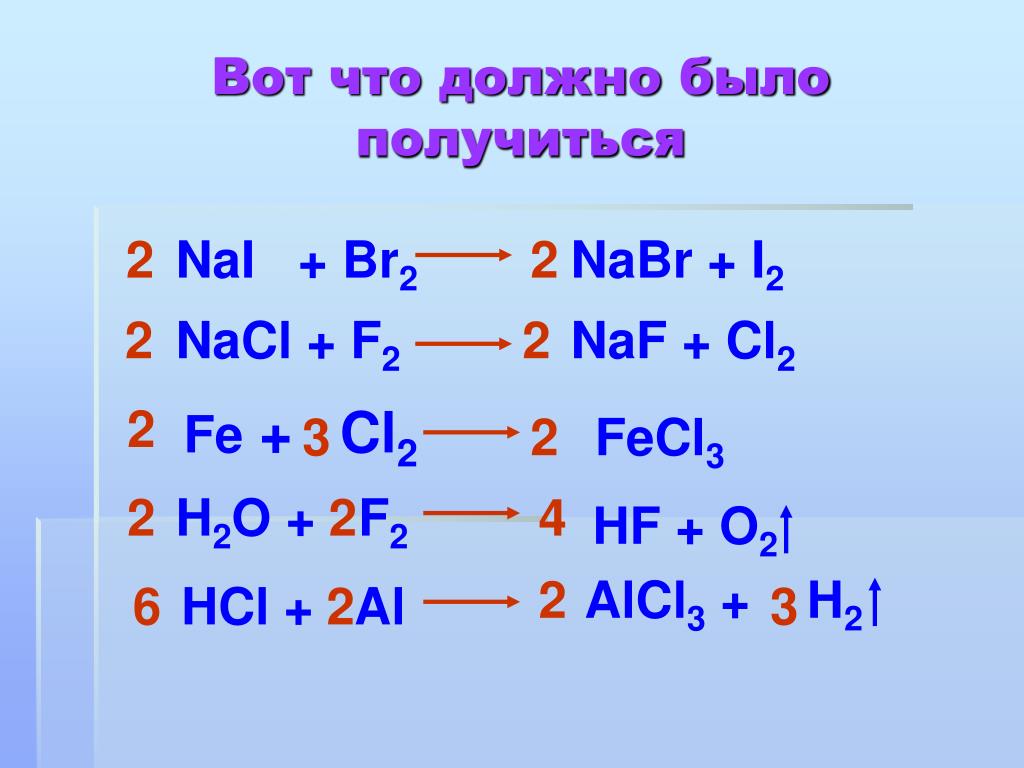
 NaBr является восстановителем, Cl2 является окислителем. Решенное и коэффициентами уравнение реакции 2 NaBr + Cl2 → 2 NaCl + Br2 с дополненными продуктами. …
NaBr является восстановителем, Cl2 является окислителем. Решенное и коэффициентами уравнение реакции 2 NaBr + Cl2 → 2 NaCl + Br2 с дополненными продуктами. …
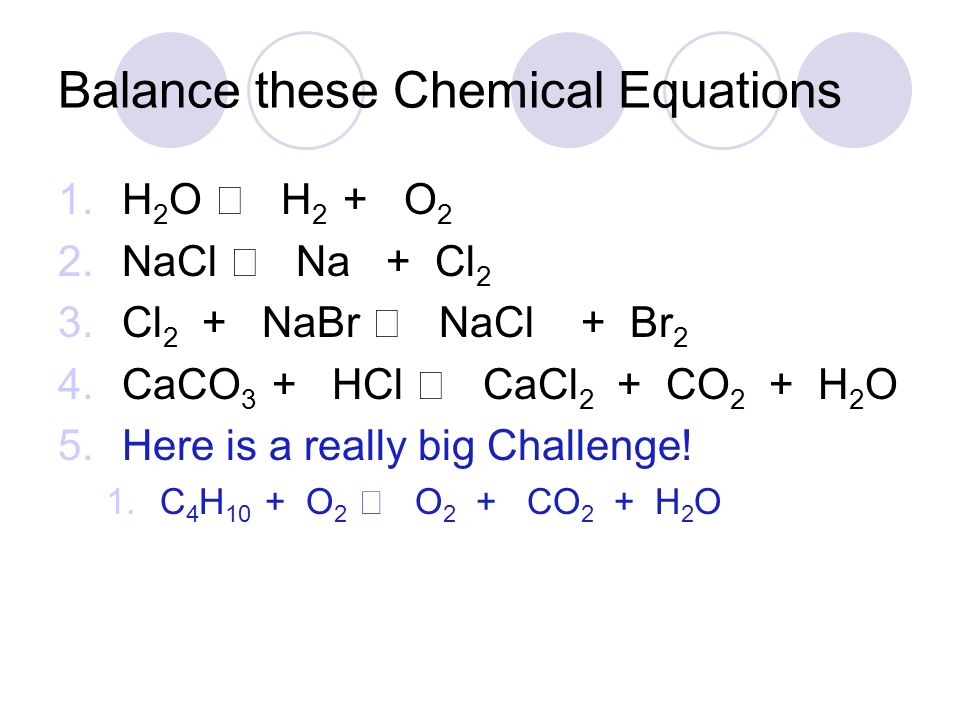
 1 Br 2 + 2 NaCl = 1 Cl 2 + 2 NaBr Cl is balanced: 2 atoms in reagents and 2 atoms in products. All atoms are now balanced and the whole equation is fully balanced:
1 Br 2 + 2 NaCl = 1 Cl 2 + 2 NaBr Cl is balanced: 2 atoms in reagents and 2 atoms in products. All atoms are now balanced and the whole equation is fully balanced:
 In this video we'll balance the equation NaBr + Cl2 = NaCl + Br2 and provide the correct coefficients for each compound..more. To balance NaBr + Cl2 = NaCl + Br2 you'll need to be sure.
In this video we'll balance the equation NaBr + Cl2 = NaCl + Br2 and provide the correct coefficients for each compound..more. To balance NaBr + Cl2 = NaCl + Br2 you'll need to be sure.
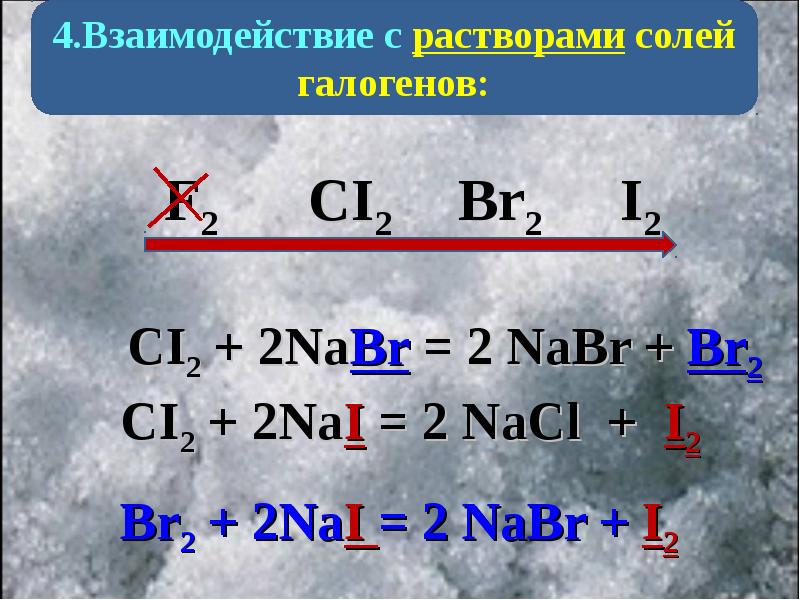
 Will a reaction occur between Br 2 and NaCl? Flexi Says: Yes, a reaction will occur. This is a single replacement reaction where bromine (Br2) can replace the chloride (Cl) in sodium …
Will a reaction occur between Br 2 and NaCl? Flexi Says: Yes, a reaction will occur. This is a single replacement reaction where bromine (Br2) can replace the chloride (Cl) in sodium …
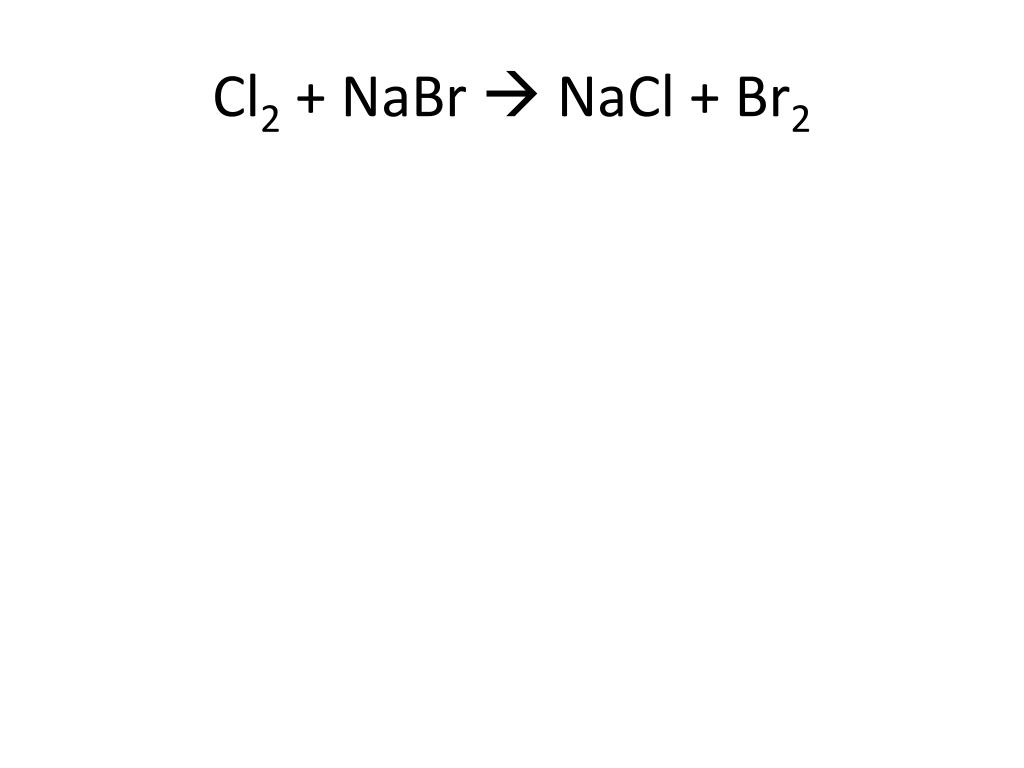 1 Br 2 + 1 NaCl = 1 Br 2 NaCl For each element, we check if the number of atoms is balanced on both sides of the equation. Br is balanced: 2 atoms in reagents and 2 atoms in products.
1 Br 2 + 1 NaCl = 1 Br 2 NaCl For each element, we check if the number of atoms is balanced on both sides of the equation. Br is balanced: 2 atoms in reagents and 2 atoms in products.
 1 NaBr + 1 Cl 2 = 1 Br 2 + 1 NaCl Для каждого элемента мы проверяем, сбалансировано ли количество атомов в обеих частях уравнения. Na сбалансирован: 1 атом в реагентах и 1 …
1 NaBr + 1 Cl 2 = 1 Br 2 + 1 NaCl Для каждого элемента мы проверяем, сбалансировано ли количество атомов в обеих частях уравнения. Na сбалансирован: 1 атом в реагентах и 1 …
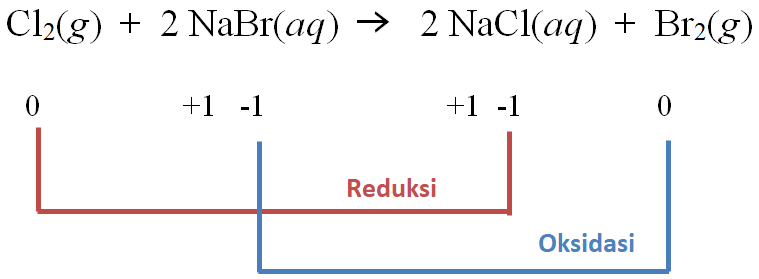 How to Balance: NaBr + Cl 2 → NaCl + Br 2 Type of Chemical Reaction: This is a single displacement reaction . Word Equation : Sodium Bromide + Chlorine gas → Sodium chloride + …
How to Balance: NaBr + Cl 2 → NaCl + Br 2 Type of Chemical Reaction: This is a single displacement reaction . Word Equation : Sodium Bromide + Chlorine gas → Sodium chloride + …
 There are three main steps for writing the net ionic equation for NaBr + Cl2 = NaCl + Br2 (Sodium bromide + Chlorine gas). First, we balance the molecular equation. Second, we write the states.
There are three main steps for writing the net ionic equation for NaBr + Cl2 = NaCl + Br2 (Sodium bromide + Chlorine gas). First, we balance the molecular equation. Second, we write the states.
 Given solubility and reactively inert solvent, BrX− B r X − (large poor nucleophile) competes with ClX− C l X − (smaller, better nucleophile) for ring-opening capture. Now factor in relative …
Given solubility and reactively inert solvent, BrX− B r X − (large poor nucleophile) competes with ClX− C l X − (smaller, better nucleophile) for ring-opening capture. Now factor in relative …
 1 Cl 2 + 1 NaBr = 1 NaCl + 1 Br 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Cl is not balanced: 2 atoms in reagents and 1 atom in products.
1 Cl 2 + 1 NaBr = 1 NaCl + 1 Br 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Cl is not balanced: 2 atoms in reagents and 1 atom in products.
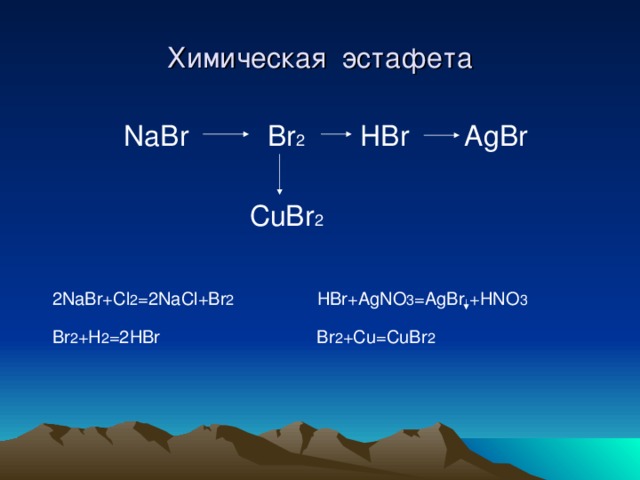
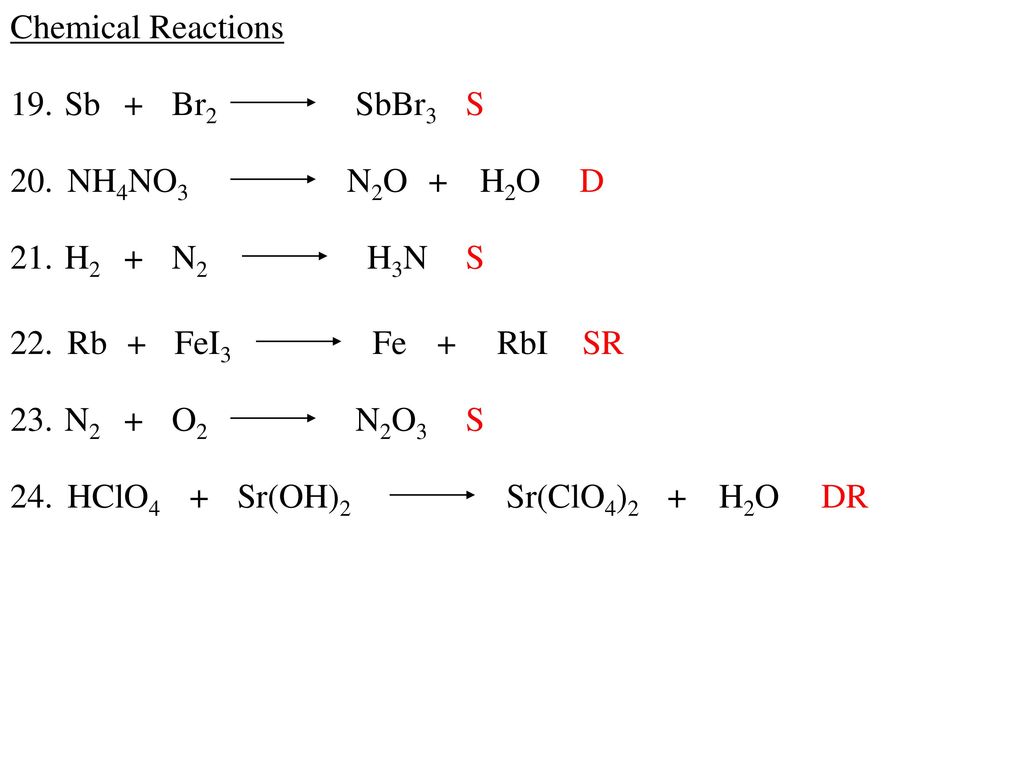
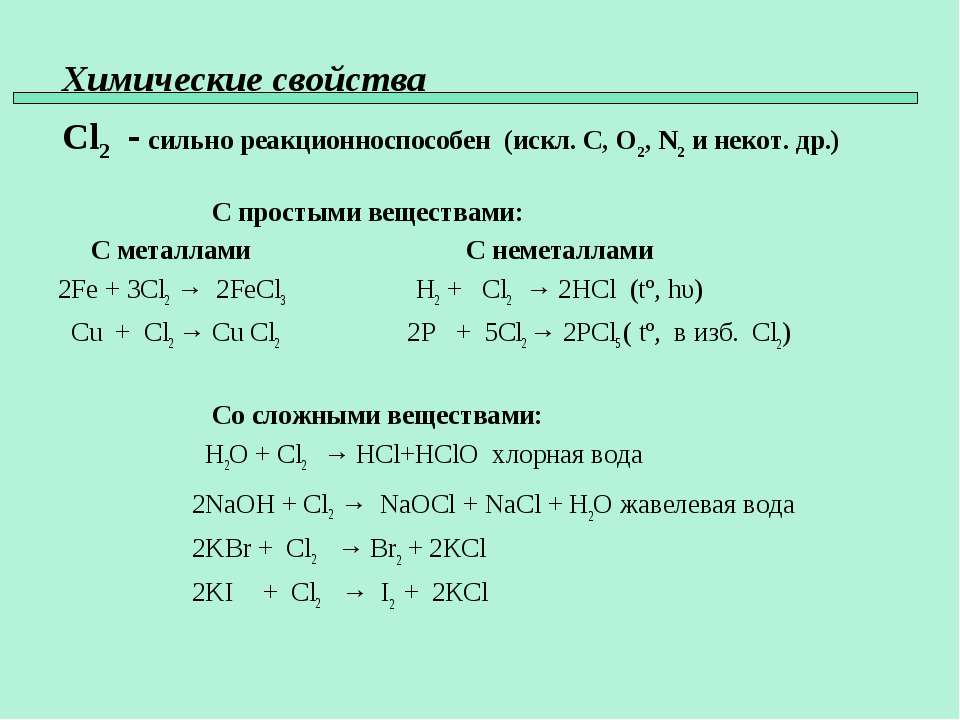
 Cl2(g) +2Br− (aq) → Br2(g) + 2Cl− (aq) The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous sodium chloride, as shown by the …
Cl2(g) +2Br− (aq) → Br2(g) + 2Cl− (aq) The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous sodium chloride, as shown by the …
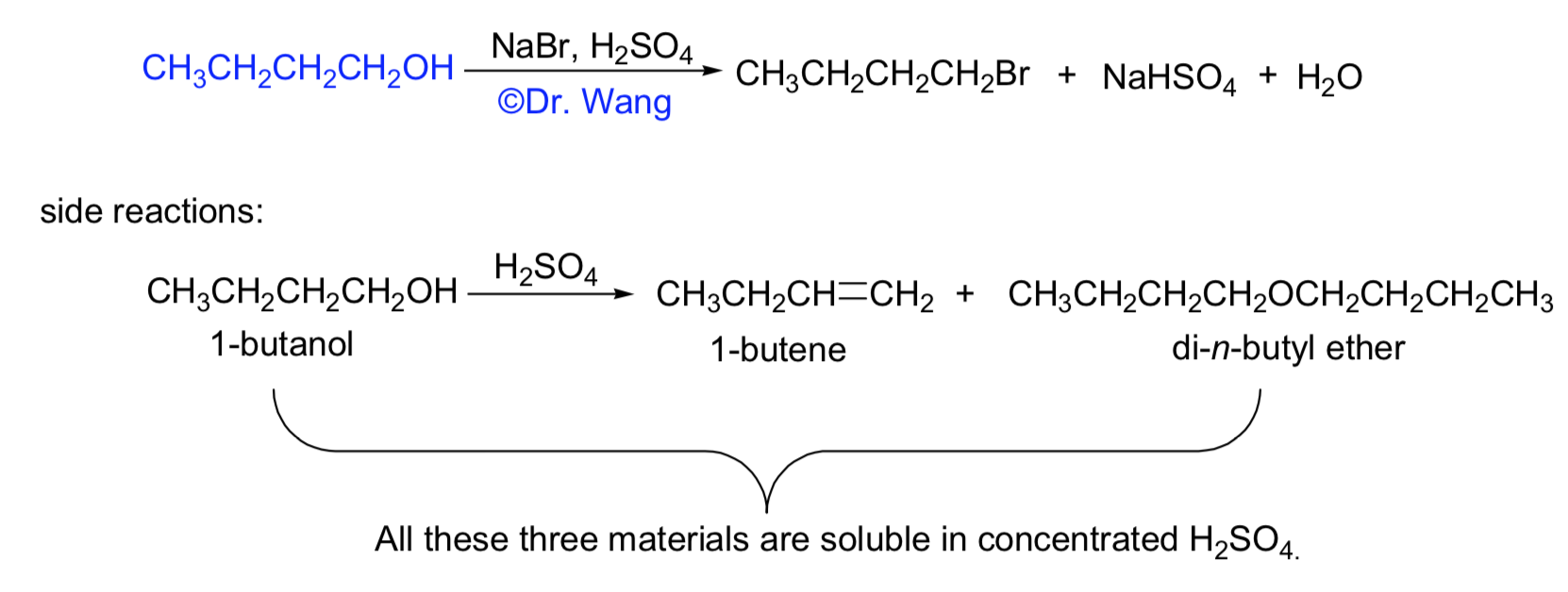
 The overall chemical equation for the alkene reaction with Br2 and NaCl is: alkene + Br2 + NaCl → alkyl dihalide + NaBr. This equation shows the addition of a bromine molecule …
The overall chemical equation for the alkene reaction with Br2 and NaCl is: alkene + Br2 + NaCl → alkyl dihalide + NaBr. This equation shows the addition of a bromine molecule …
 1 Br 2 + 1 NaCl = 1 BrNaCl For each element, we check if the number of atoms is balanced on both sides of the equation. Br is not balanced: 2 atoms in reagents and 1 atom in products.
1 Br 2 + 1 NaCl = 1 BrNaCl For each element, we check if the number of atoms is balanced on both sides of the equation. Br is not balanced: 2 atoms in reagents and 1 atom in products.
 1 NaCl + 1 Br 2 = 1 NaClBr For each element, we check if the number of atoms is balanced on both sides of the equation. Na is balanced: 1 atom in reagents and 1 atom in products.
1 NaCl + 1 Br 2 = 1 NaClBr For each element, we check if the number of atoms is balanced on both sides of the equation. Na is balanced: 1 atom in reagents and 1 atom in products.
Еще по теме:










Еще по теме: