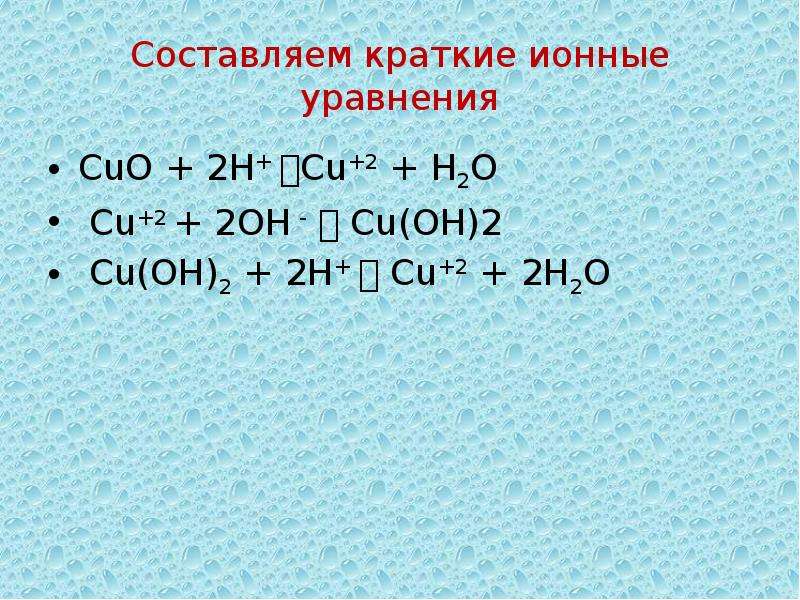
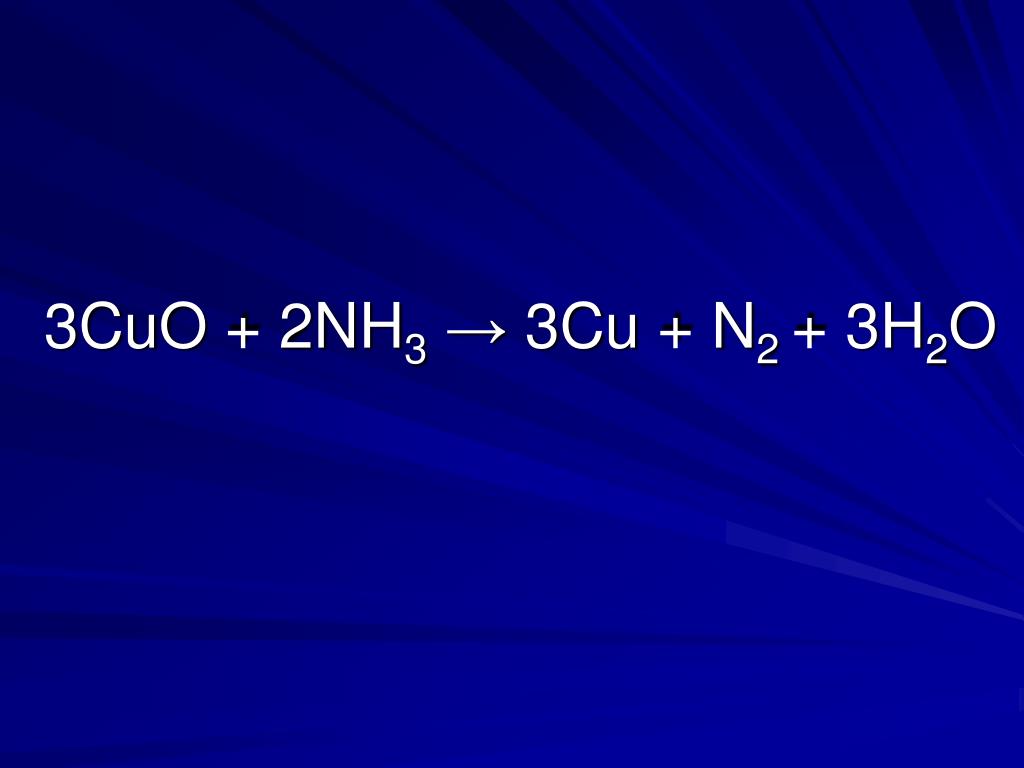
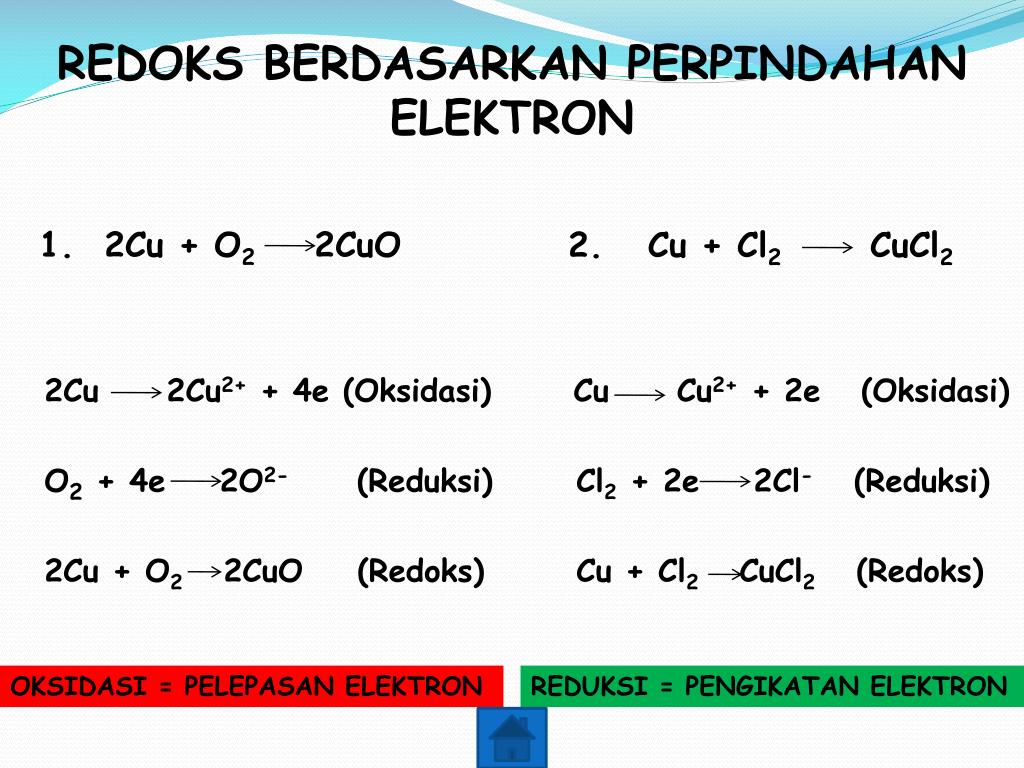
 Это окислительно-восстановительная (редокс) реакция: Cu является восстановителем, O2 является окислителем. Решенное и коэффициентами уравнение реакции Cu + O2 → …
Это окислительно-восстановительная (редокс) реакция: Cu является восстановителем, O2 является окислителем. Решенное и коэффициентами уравнение реакции Cu + O2 → …
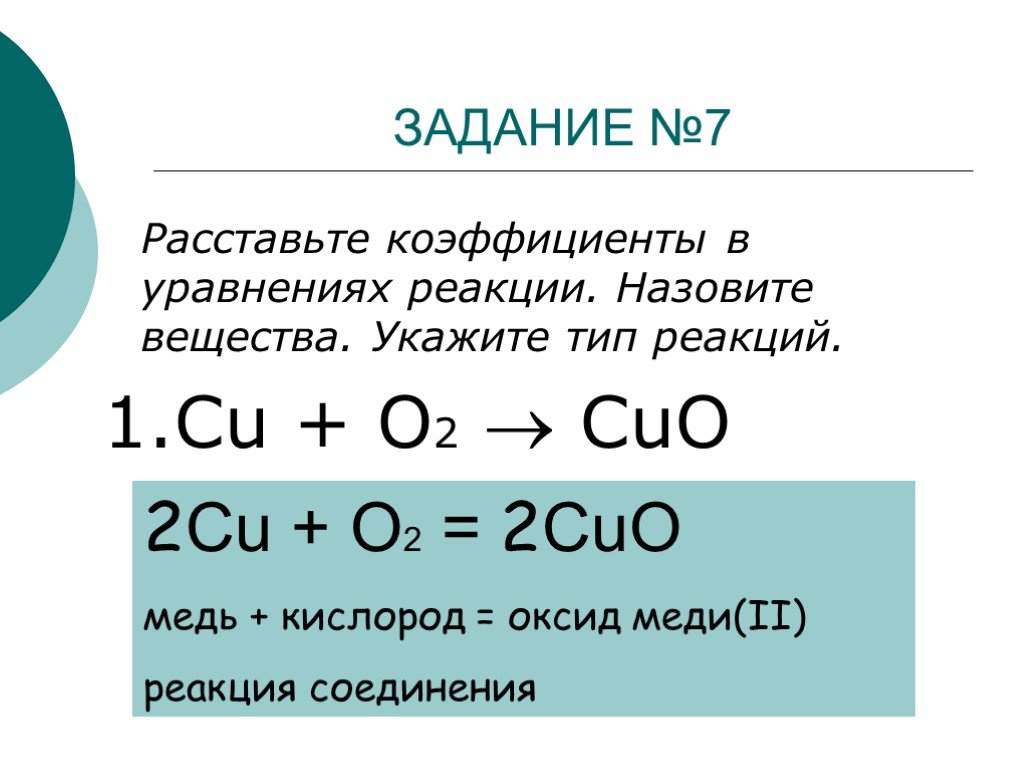
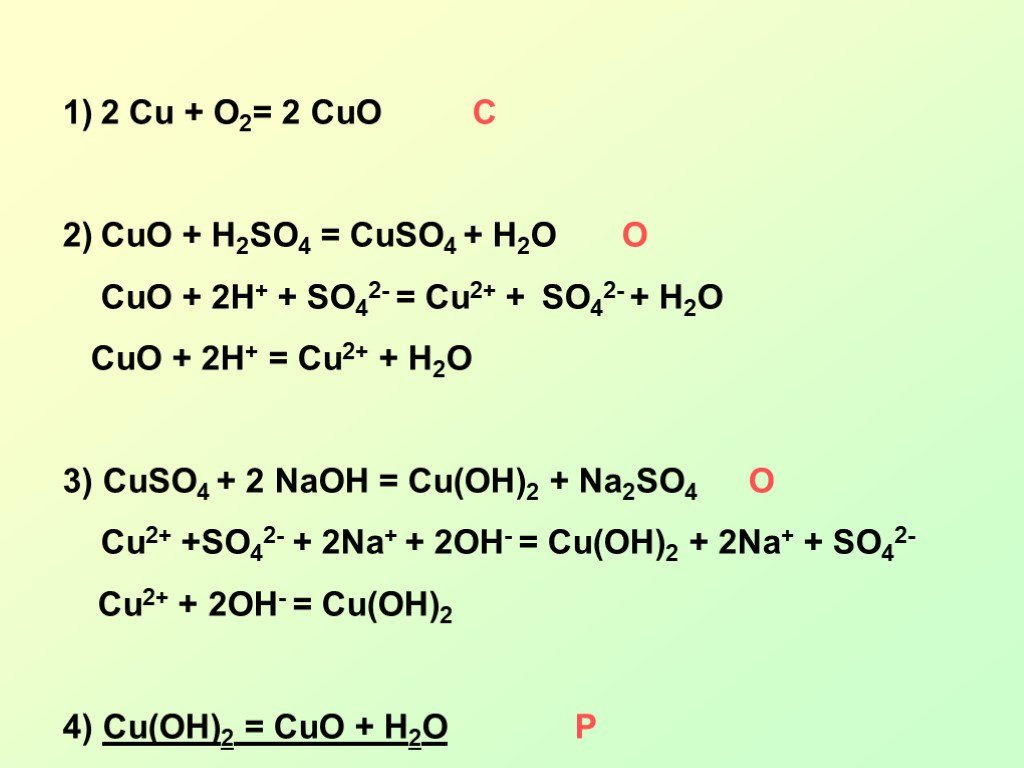
 Решенное и коэффициентами уравнение реакции 2 Cu + O2 → 2 CuO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 Cu + O2 → 2 CuO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Cu + O2 = CuO is a Synthesis reaction where two moles of Copper [Cu] and one mole of Dioxygen [O 2] combine to form two moles of Copper(Ii) Oxide [CuO]
Cu + O2 = CuO is a Synthesis reaction where two moles of Copper [Cu] and one mole of Dioxygen [O 2] combine to form two moles of Copper(Ii) Oxide [CuO]
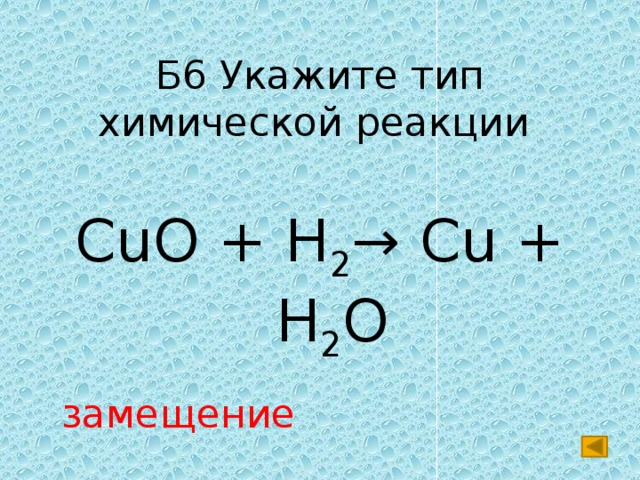
 Cu is balanced: 1 atom in reagents and 1 atom in products. O is not balanced: 2 atoms in reagents and 1 atom in products. In order to balance O on both sides we:
Cu is balanced: 1 atom in reagents and 1 atom in products. O is not balanced: 2 atoms in reagents and 1 atom in products. In order to balance O on both sides we:
 Cu2 + O2 = CuO is a Synthesis reaction where one mole of Dinuclear Copper Ion [Cu 2] and one mole of Dioxygen [O 2] combine to form two moles of Copper(Ii) Oxide [CuO]
Cu2 + O2 = CuO is a Synthesis reaction where one mole of Dinuclear Copper Ion [Cu 2] and one mole of Dioxygen [O 2] combine to form two moles of Copper(Ii) Oxide [CuO]
 In this video we determine the type of chemical reaction for the equation Cu + O2 = CuO (Copper + Oxygen gas)..more. Since we have a two substances combining, Cu + O2 = CuO is a Synthesis.
In this video we determine the type of chemical reaction for the equation Cu + O2 = CuO (Copper + Oxygen gas)..more. Since we have a two substances combining, Cu + O2 = CuO is a Synthesis.
 To balance Cu + O2 = CuO you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change the coefficients.
To balance Cu + O2 = CuO you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change the coefficients.
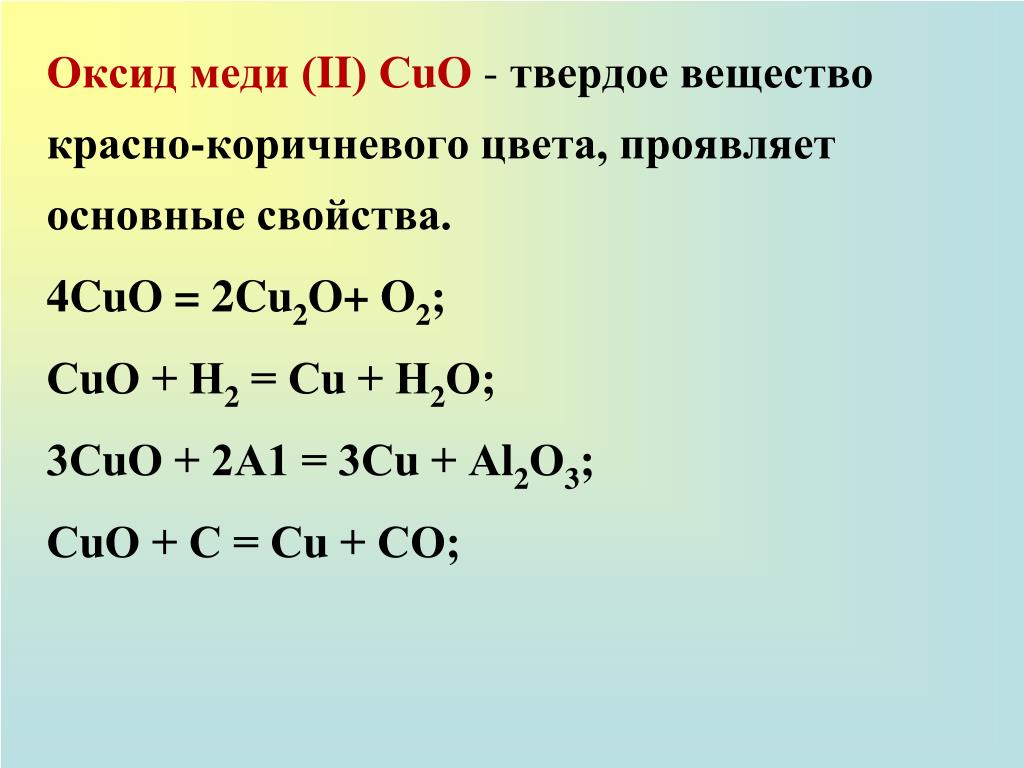
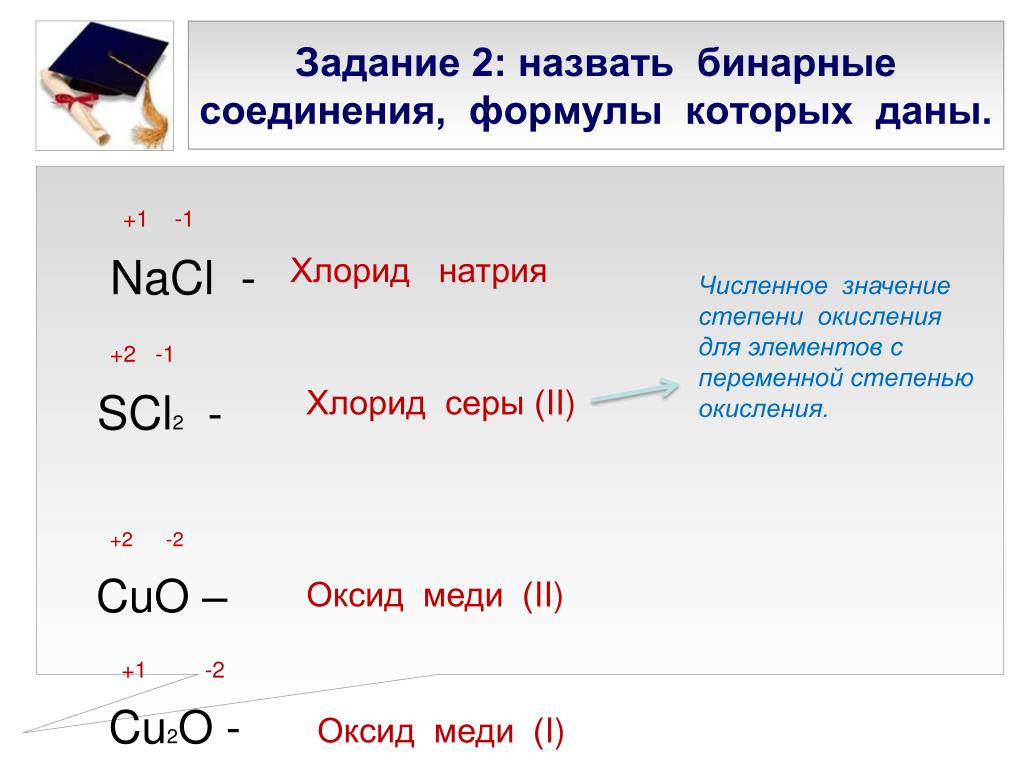
 Copper (II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu 2 O or copper (I) oxide (cuprous …
Copper (II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu 2 O or copper (I) oxide (cuprous …
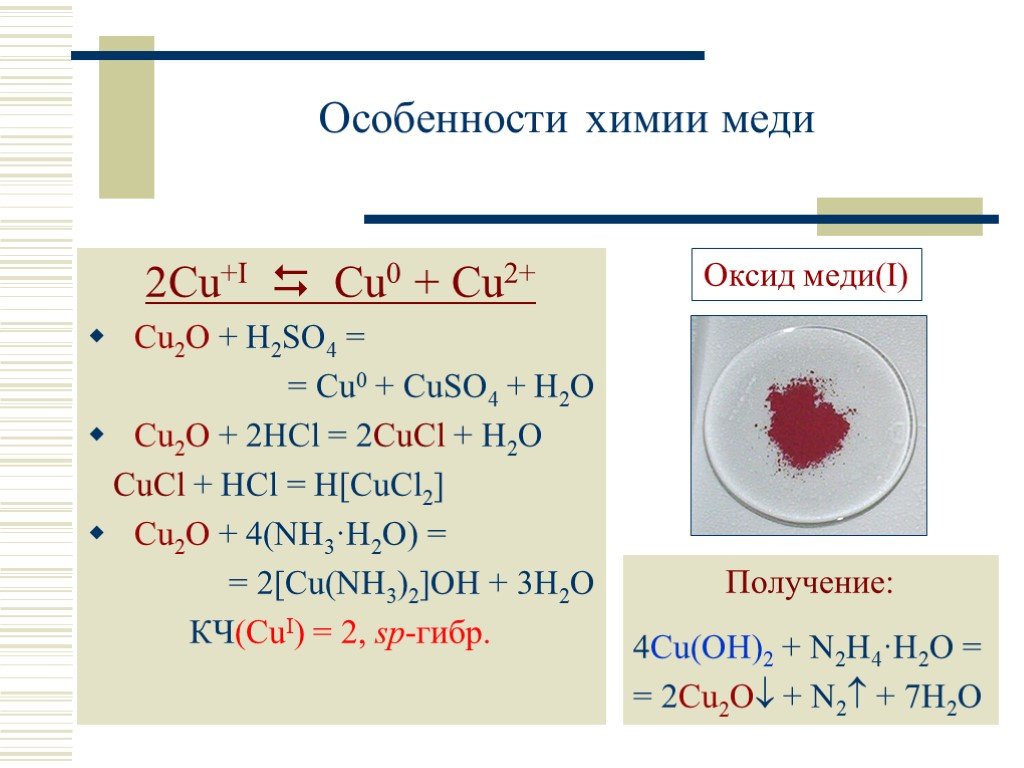 1 CU(s) + 1 O 2 (g) = 1 CUO(s) For each element, we check if the number of atoms is balanced on both sides of the equation. C is balanced: 1 atom in reagents and 1 atom in products.
1 CU(s) + 1 O 2 (g) = 1 CUO(s) For each element, we check if the number of atoms is balanced on both sides of the equation. C is balanced: 1 atom in reagents and 1 atom in products.
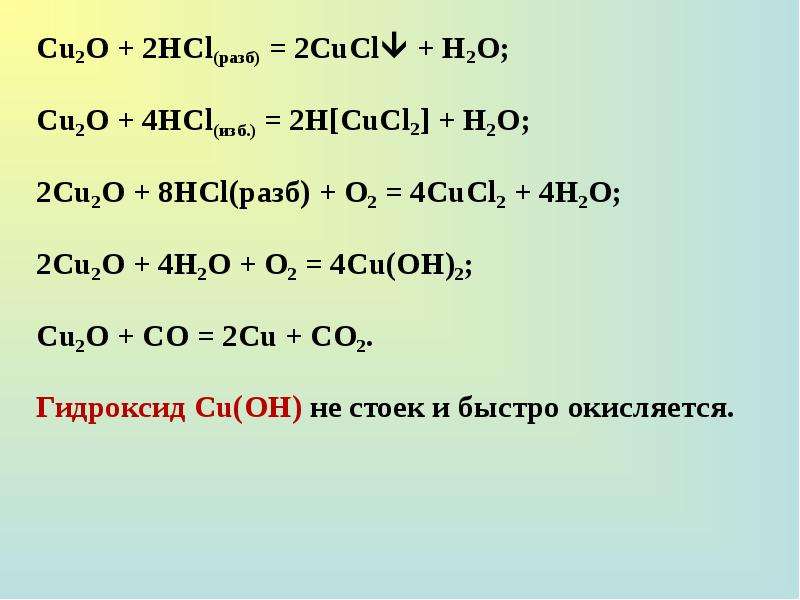
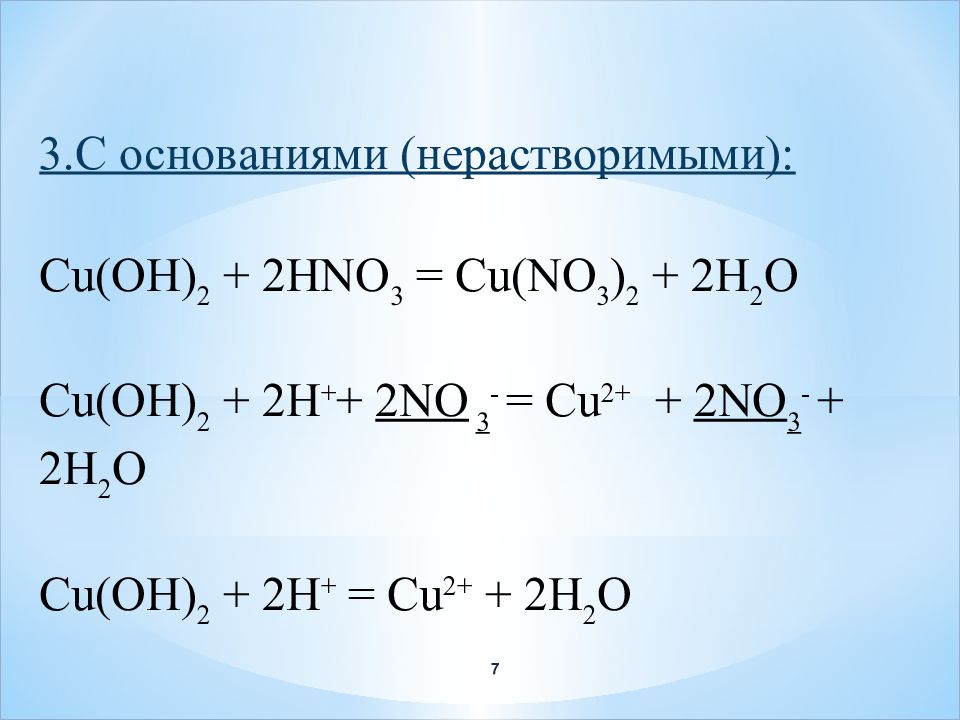
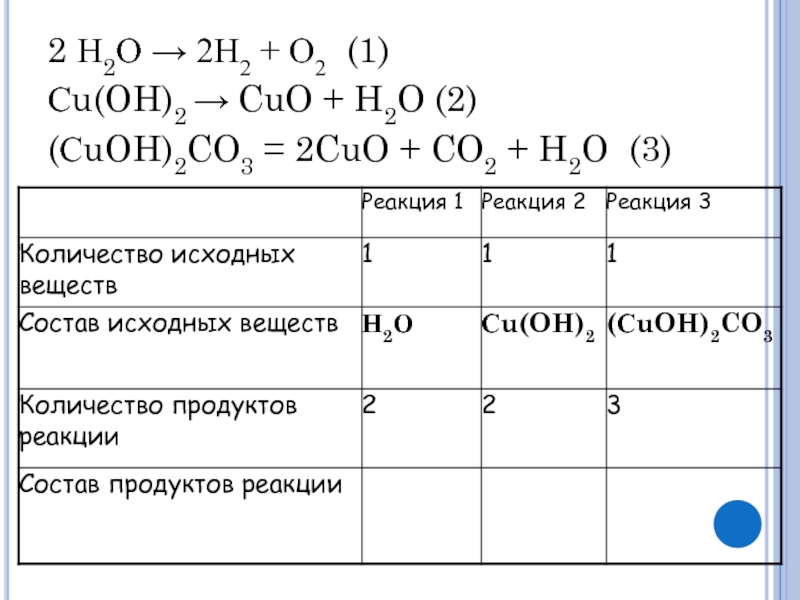
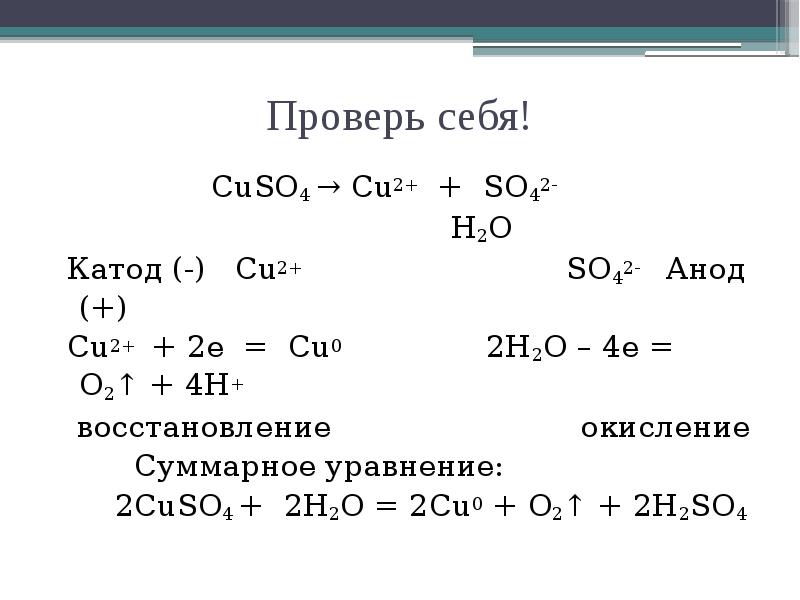
 Решенное и коэффициентами уравнение реакции 2 Cu2O + O2 → 4 CuO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 Cu2O + O2 → 4 CuO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Бесплатный калькулятор Химических Реакций - пошаговый расчет химических реакций
Бесплатный калькулятор Химических Реакций - пошаговый расчет химических реакций
 Cu + O2= en. Related Symbolab blog posts. My Notebook, the Symbolab way. Math notebooks have been around for hundreds of years. You write down problems, solutions and notes to go …
Cu + O2= en. Related Symbolab blog posts. My Notebook, the Symbolab way. Math notebooks have been around for hundreds of years. You write down problems, solutions and notes to go …
 1 Cu + 1 O 2 = 1 Cu 2 O 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Cu is not balanced: 1 atom in reagents and 2 atoms in products.
1 Cu + 1 O 2 = 1 Cu 2 O 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Cu is not balanced: 1 atom in reagents and 2 atoms in products.
 In this study, CuCr 2 O 4-based catalytic oxygen carriers are tailored for lattice oxygen participating in low-temperature methanol reforming. We found that the low …
In this study, CuCr 2 O 4-based catalytic oxygen carriers are tailored for lattice oxygen participating in low-temperature methanol reforming. We found that the low …
 Here, we study the strategy for merging electrodissolution with catalysis to skip these extra steps and demonstrate efficient waste-minimized transformations to access Cu …
Here, we study the strategy for merging electrodissolution with catalysis to skip these extra steps and demonstrate efficient waste-minimized transformations to access Cu …
 Absolute concentrations of 19 macro and trace elements (Ag, Al, B, Ba, Ca, Cd, Cu, Fe, K, Mg, Mn, Na, P, Pb, S, Si, Sr, Tl, Zn) were determined using inductively coupled plasma optical …
Absolute concentrations of 19 macro and trace elements (Ag, Al, B, Ba, Ca, Cd, Cu, Fe, K, Mg, Mn, Na, P, Pb, S, Si, Sr, Tl, Zn) were determined using inductively coupled plasma optical …
Еще по теме:










Еще по теме: