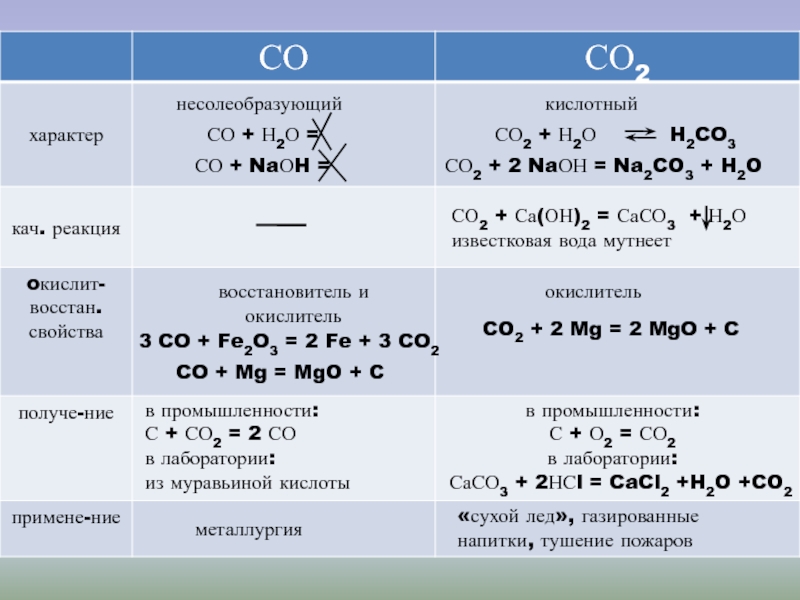
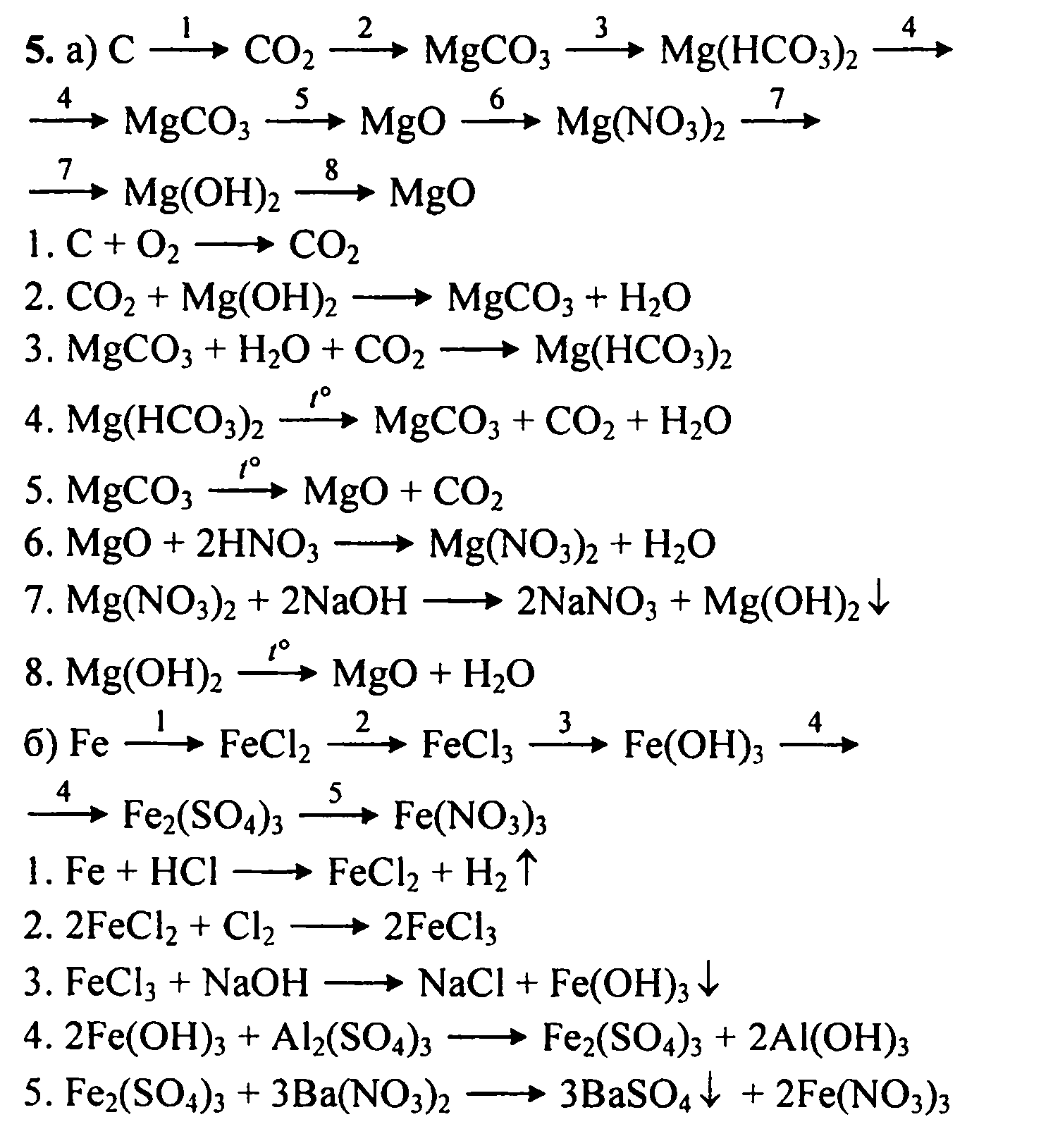
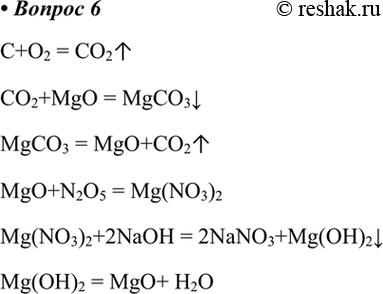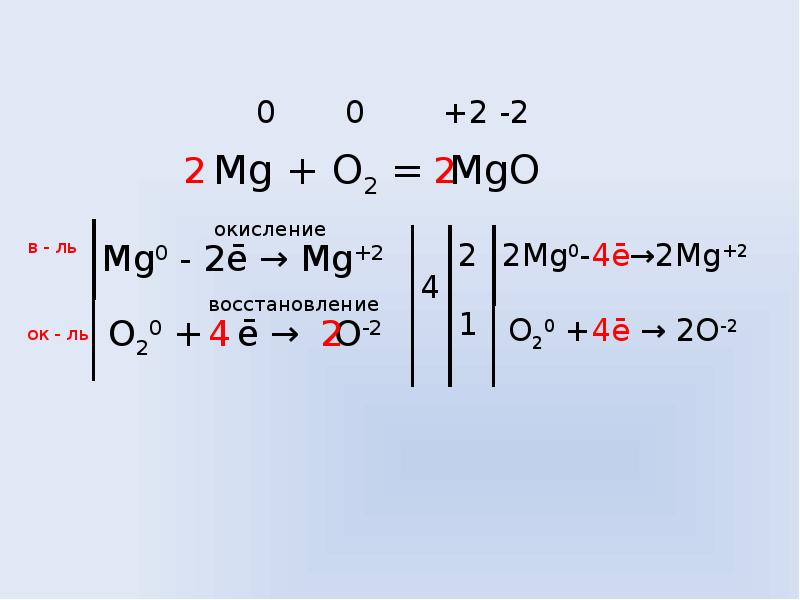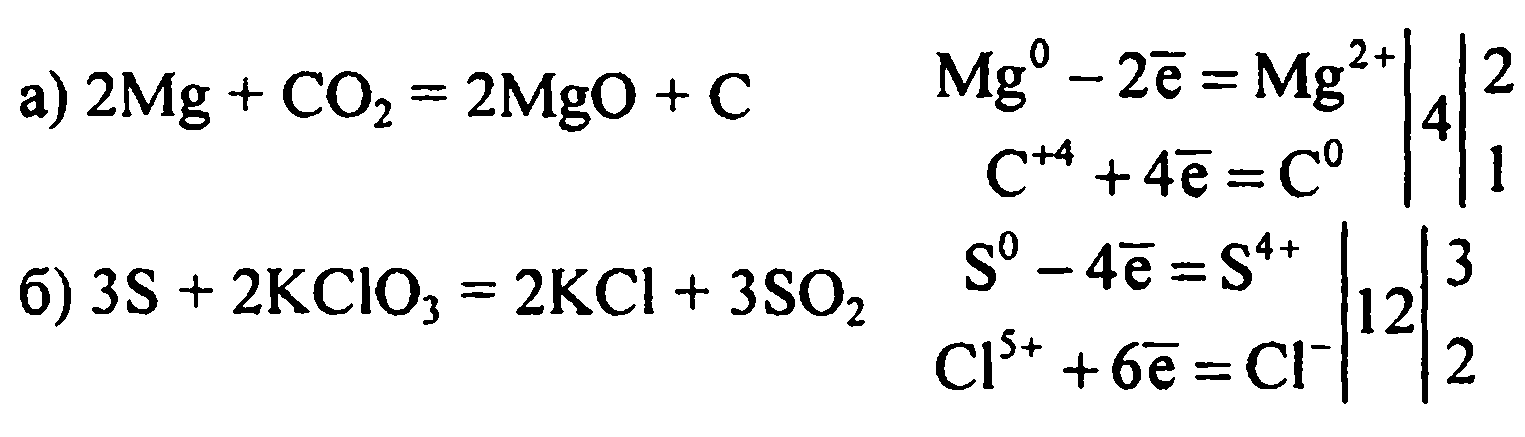 Решенное и коэффициентами уравнение реакции CO2 + 2 Mg → 2 MgO + C с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции CO2 + 2 Mg → 2 MgO + C с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 1 Mg + 1 CO 2 = 1 MgO + 1 C For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in products. C is …
1 Mg + 1 CO 2 = 1 MgO + 1 C For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in products. C is …
 Complete the following blank in the equation as indicated. Give word equation for the following chemical reaction and give the names of the product formed.
Complete the following blank in the equation as indicated. Give word equation for the following chemical reaction and give the names of the product formed.
 In this video we'll balance the equation Mg + CO2 = MgO + C and provide the correct coefficients for each compound. To balance Mg + CO2 = MgO + C you'll need to be …
In this video we'll balance the equation Mg + CO2 = MgO + C and provide the correct coefficients for each compound. To balance Mg + CO2 = MgO + C you'll need to be …
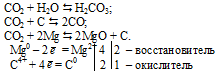 1.In the reaction, 2 M g + C O 2 → 2 M g O + C, M g is in its 0 oxidation state and C shows + 4 oxidation state in C O 2 molecule before the reaction. 2.After the reaction, M g loses 2 e-and …
1.In the reaction, 2 M g + C O 2 → 2 M g O + C, M g is in its 0 oxidation state and C shows + 4 oxidation state in C O 2 molecule before the reaction. 2.After the reaction, M g loses 2 e-and …
 Carbon dioxide gas reacts with Mg metal to form magnesium oxide along with C. The balanced chemical equation of the above-given reaction is written as follows- In this …
Carbon dioxide gas reacts with Mg metal to form magnesium oxide along with C. The balanced chemical equation of the above-given reaction is written as follows- In this …
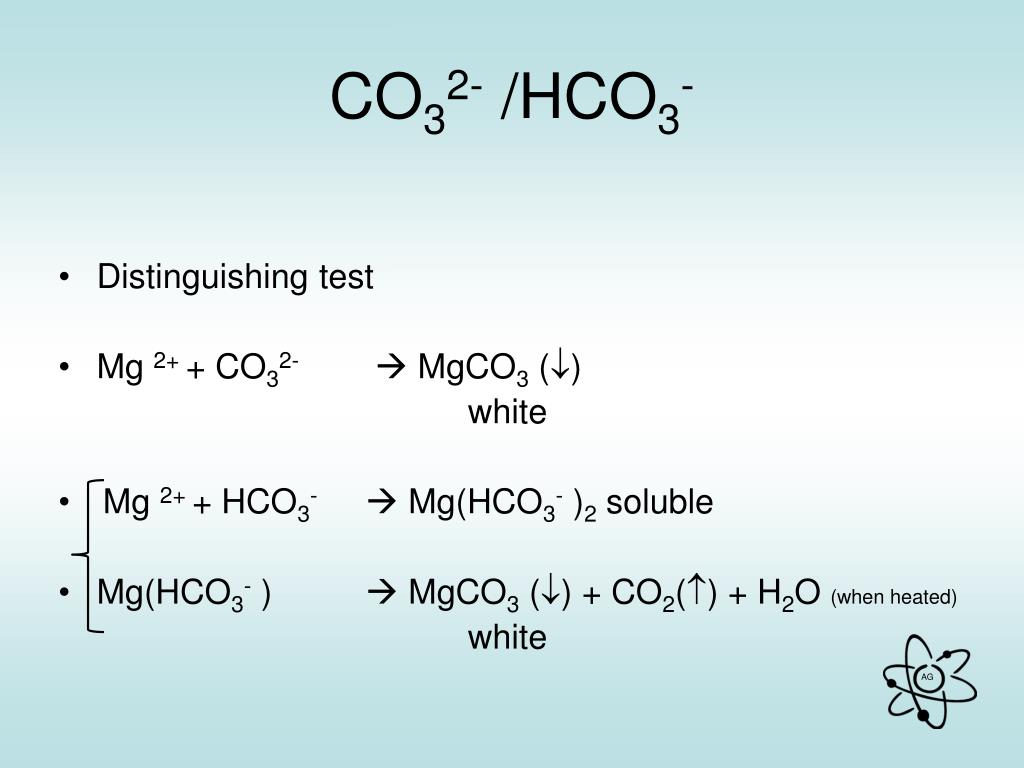
 Mg+CO2= MgO + C проставляем степени окисления: на mg 0, на С +4, на О -2. После стрелки- на mg +2, на O -2, на С 0.
Mg+CO2= MgO + C проставляем степени окисления: на mg 0, на С +4, на О -2. После стрелки- на mg +2, на O -2, на С 0.
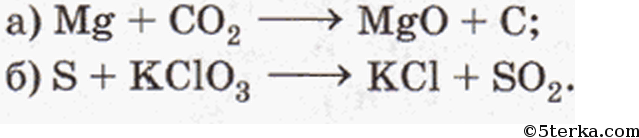 1 Mg + 1 CO 2 = 1 MgO + 1 C 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in products. C is …
1 Mg + 1 CO 2 = 1 MgO + 1 C 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in products. C is …
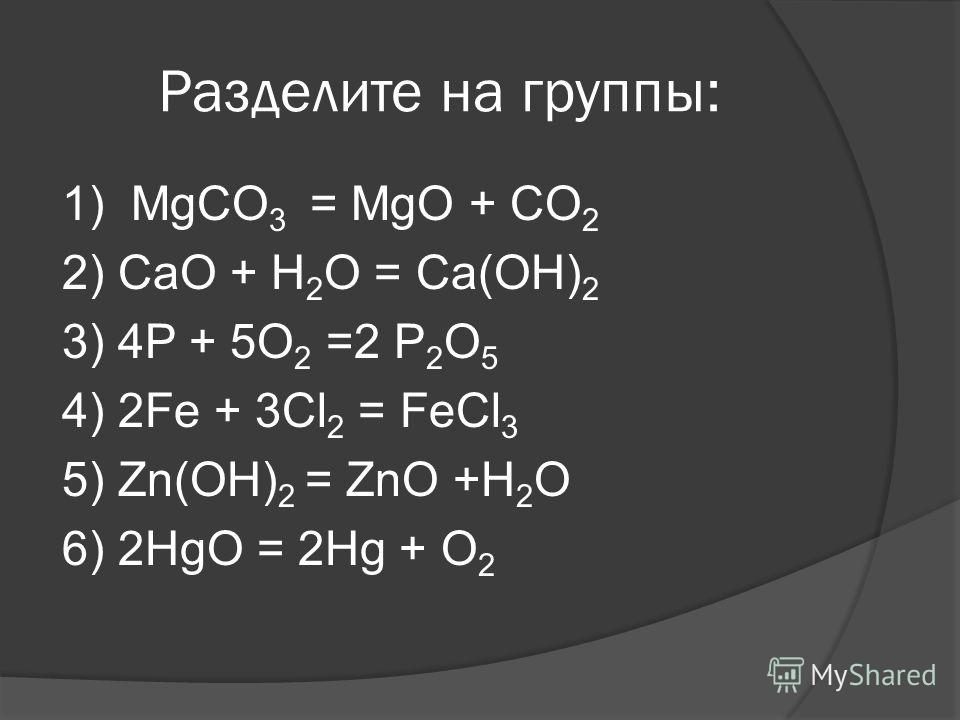
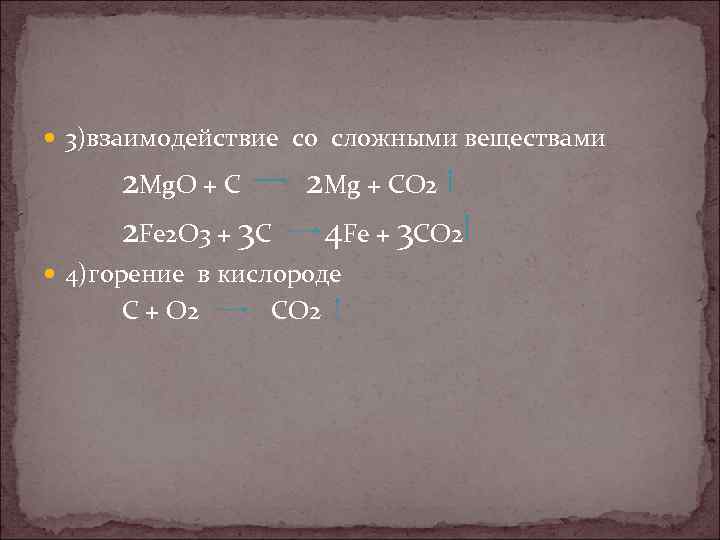 Решенное и коэффициентами уравнение реакции MgCO3 → MgO + CO2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции MgCO3 → MgO + CO2 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
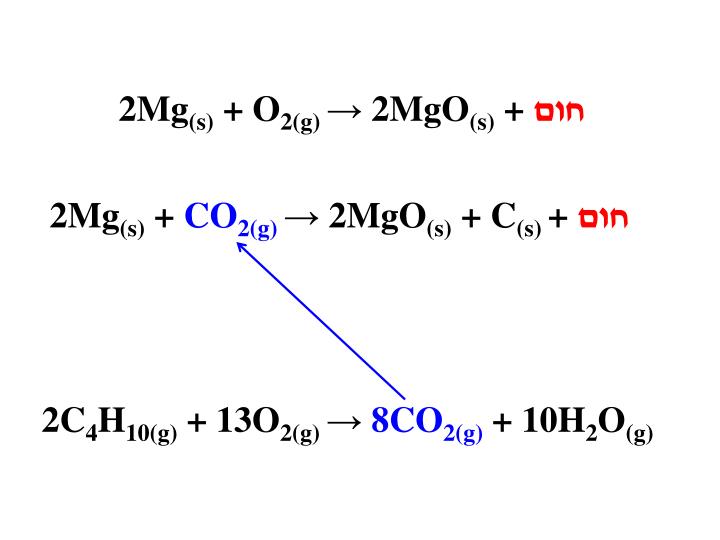 The reaction we are going to demonstrate here involves the extremely exothermic (heat evolving) and thermodynamically favorable reaction of magnesium metal (Mg) with carbon dioxide: 2 Mg(s) + CO 2 2 MgO(s) + C(s) …
The reaction we are going to demonstrate here involves the extremely exothermic (heat evolving) and thermodynamically favorable reaction of magnesium metal (Mg) with carbon dioxide: 2 Mg(s) + CO 2 2 MgO(s) + C(s) …
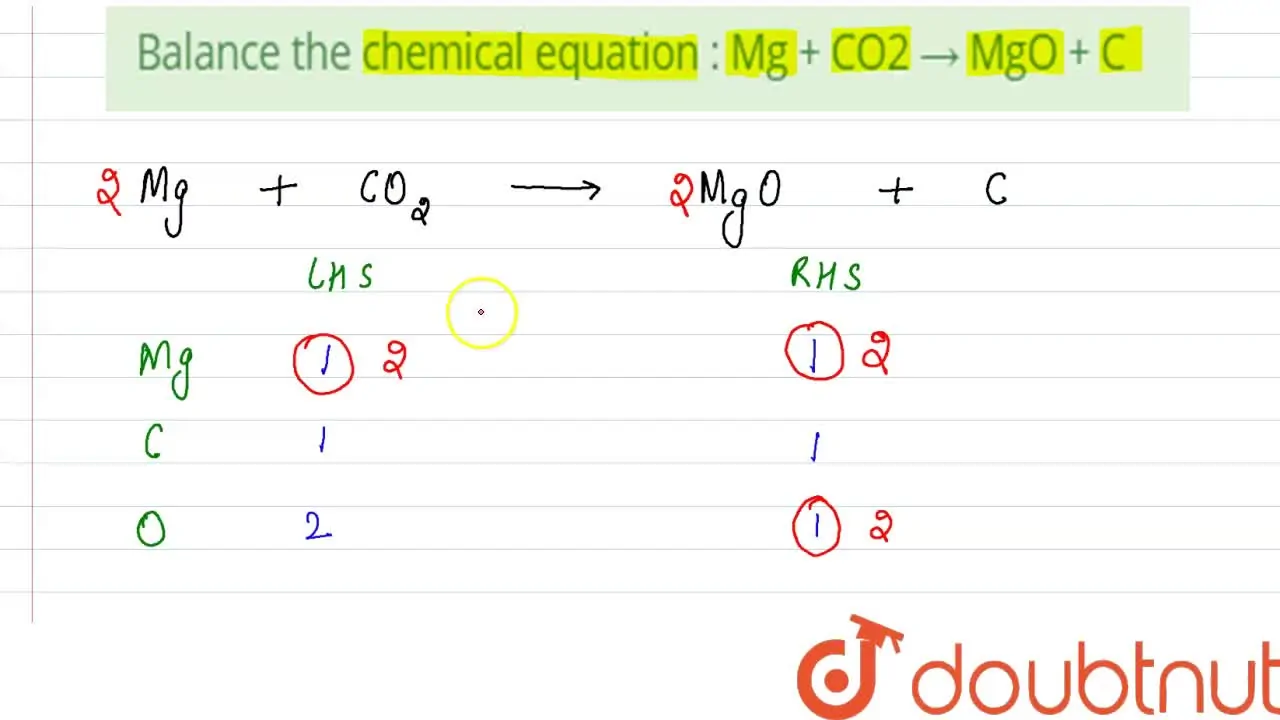 MgO + C2 = Mg + CO is a Single Displacement (Substitution) reaction where two moles of Magnesium Oxide [MgO] and one mole of Dicarbon [C 2] react to form two moles of …
MgO + C2 = Mg + CO is a Single Displacement (Substitution) reaction where two moles of Magnesium Oxide [MgO] and one mole of Dicarbon [C 2] react to form two moles of …
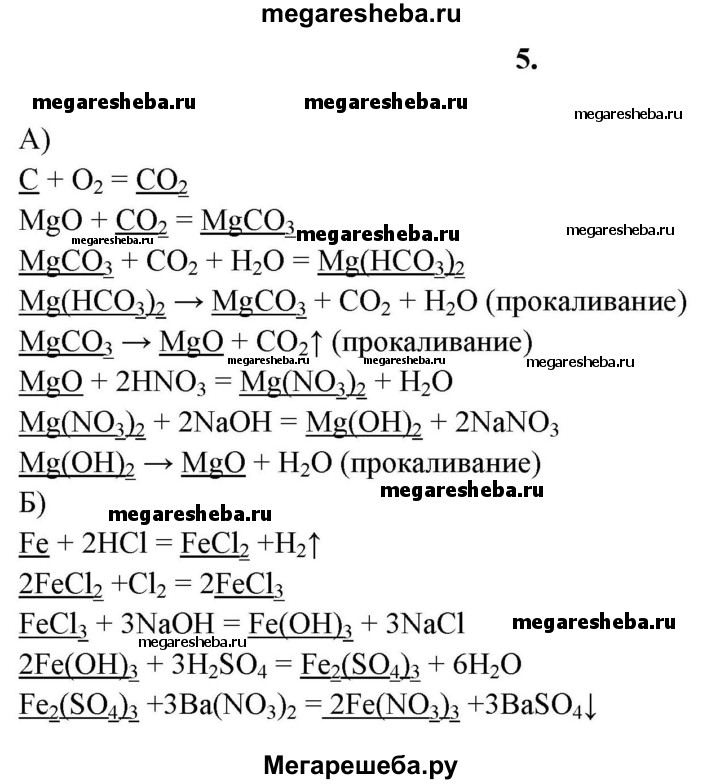
 Решенное и коэффициентами уравнение реакции CO2 + MgO → MgCO3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции CO2 + MgO → MgCO3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 1 Mg(s) + 1 CO 2 (g) = 1 MgO(s) + 1 C(s) For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in …
1 Mg(s) + 1 CO 2 (g) = 1 MgO(s) + 1 C(s) For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in …
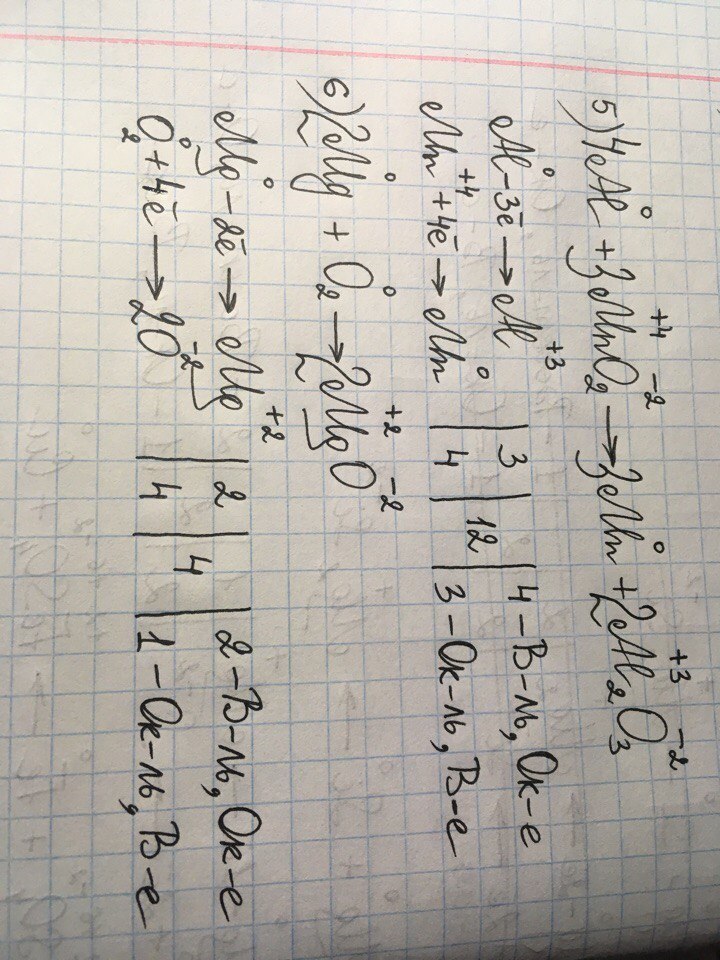 Abstract. Carbonate looping using MgO-based materials has recently ignited scientific interest for CO 2 capture at intermediate temperatures (275–375 °C), with the main limitation being the …
Abstract. Carbonate looping using MgO-based materials has recently ignited scientific interest for CO 2 capture at intermediate temperatures (275–375 °C), with the main limitation being the …
 Решенное и коэффициентами уравнение реакции MgCO3 → CO2 + MgO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции MgCO3 → CO2 + MgO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
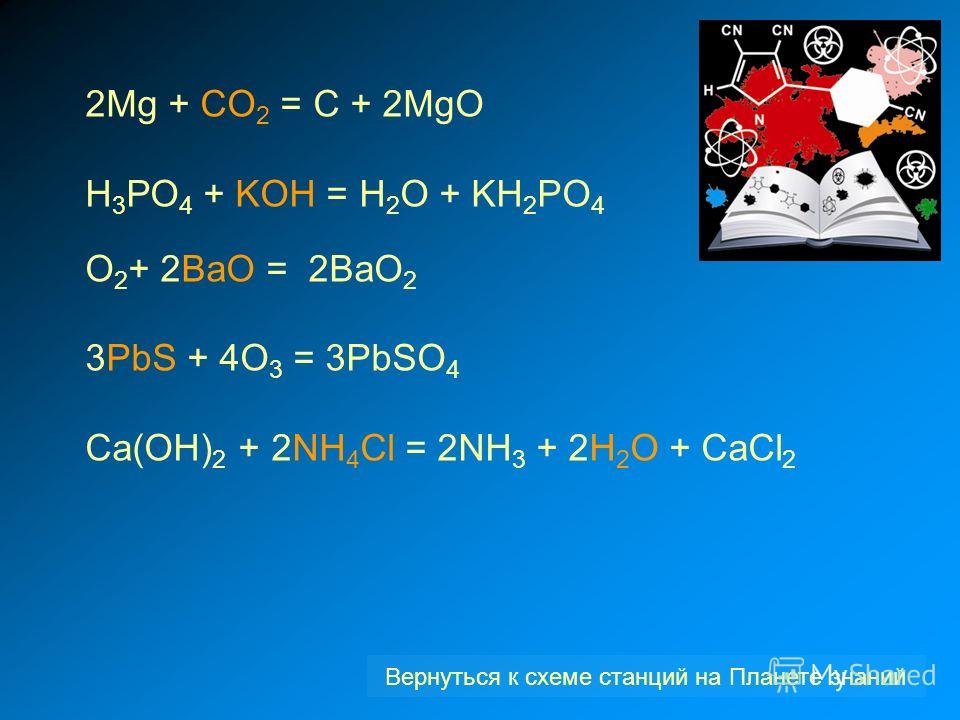
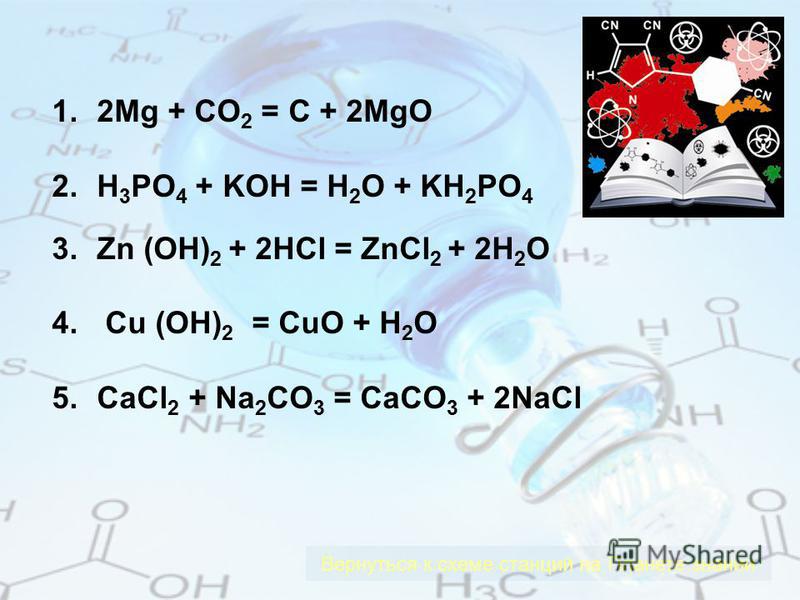 1 CO 2 + 1 Mg = 1 C + 1 MgO For each element, we check if the number of atoms is balanced on both sides of the equation. C is balanced: 1 atom in reagents and 1 atom in products. O is not …
1 CO 2 + 1 Mg = 1 C + 1 MgO For each element, we check if the number of atoms is balanced on both sides of the equation. C is balanced: 1 atom in reagents and 1 atom in products. O is not …
Еще по теме:









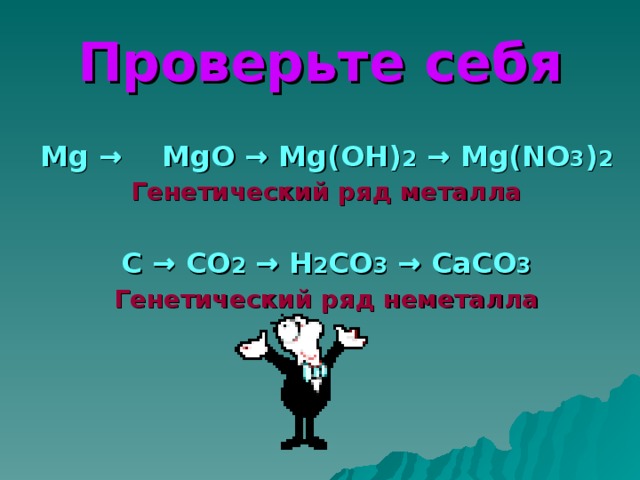



Еще по теме:


.PNG)