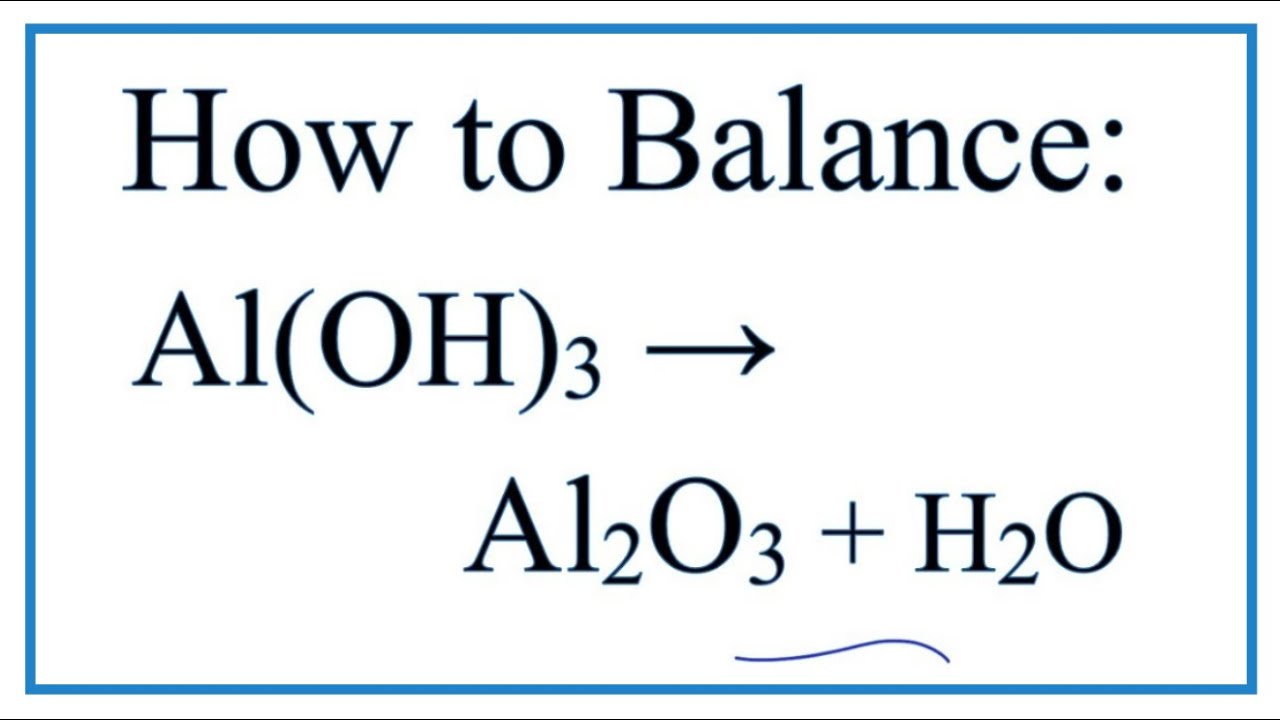
Решенное и коэффициентами уравнение реакции 2 Al(OH)3 → Al2O3 + 3 H2O с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 Al(OH)3 → Al2O3 + 3 H2O с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
 Al2O3 + H2O = (Al)(OH)3 is a Synthesis reaction where one mole of Aluminum Oxide [Al 2 O 3] and three moles of Water [H 2 O] combine to form two moles of Aluminum Hydroxide [(Al)(OH) 3]
Al2O3 + H2O = (Al)(OH)3 is a Synthesis reaction where one mole of Aluminum Oxide [Al 2 O 3] and three moles of Water [H 2 O] combine to form two moles of Aluminum Hydroxide [(Al)(OH) 3]
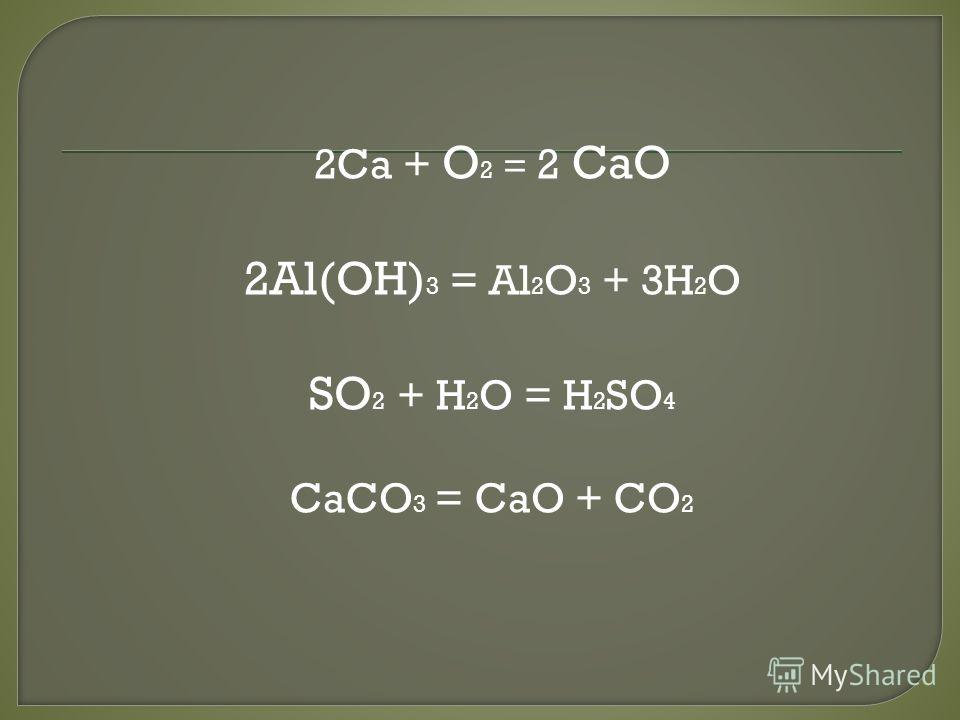
 2Al(OH) 3 = Al 2 O 3 + 3H 2 O. Данная реакция называется реакцией разложения. Вещество гидроксид алюминия разлагается на оксид алюминия и воду.
2Al(OH) 3 = Al 2 O 3 + 3H 2 O. Данная реакция называется реакцией разложения. Вещество гидроксид алюминия разлагается на оксид алюминия и воду.
 Solution. Balanced chemical equation: A balanced chemical equation represent the equation in which the number of atom of each element is equal on both the reactant and product side. …
Solution. Balanced chemical equation: A balanced chemical equation represent the equation in which the number of atom of each element is equal on both the reactant and product side. …
 2 Al(OH)3 → Al2O3 + 3 H2O Реакция термического разложения гидроксида алюминия с образованием оксида алюминия и воды. Данная реакция протекает при температуре …
2 Al(OH)3 → Al2O3 + 3 H2O Реакция термического разложения гидроксида алюминия с образованием оксида алюминия и воды. Данная реакция протекает при температуре …
 In this video we determine the type of chemical reaction for the equation Al(OH)3 = Al2O3 + H2O (Aluminum hydroxide (at high temperatures)).Since we one subs.
In this video we determine the type of chemical reaction for the equation Al(OH)3 = Al2O3 + H2O (Aluminum hydroxide (at high temperatures)).Since we one subs.
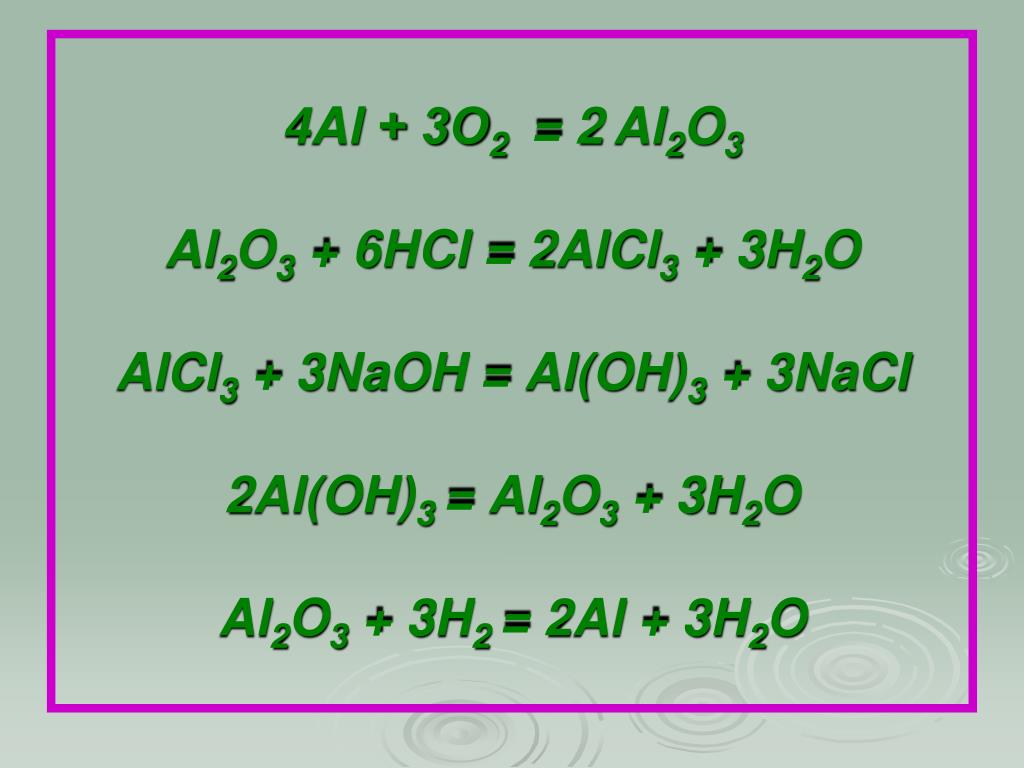
 Al(OH)3=Al2O3+H2O Внимательно смотрим на реакцию. Слева алюминий один, а справа алюминия два. Перед алюминием ставим 2.
Al(OH)3=Al2O3+H2O Внимательно смотрим на реакцию. Слева алюминий один, а справа алюминия два. Перед алюминием ставим 2.
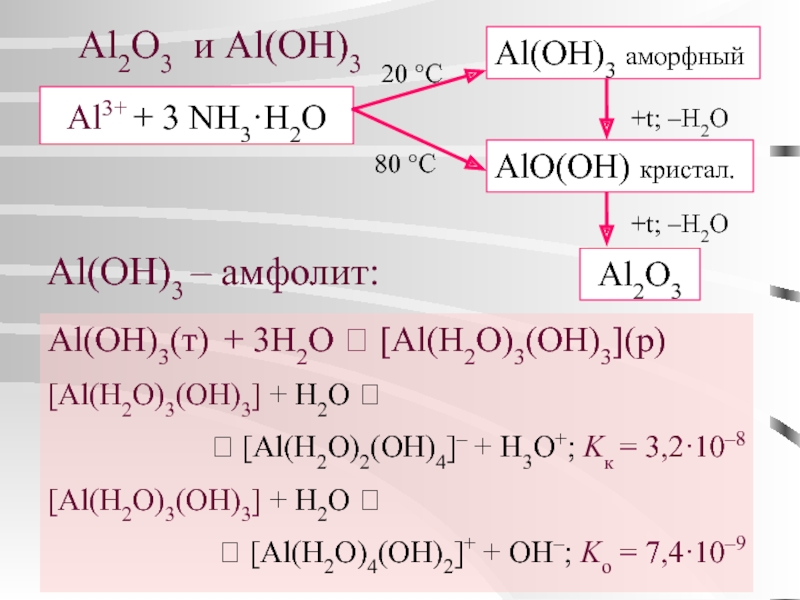
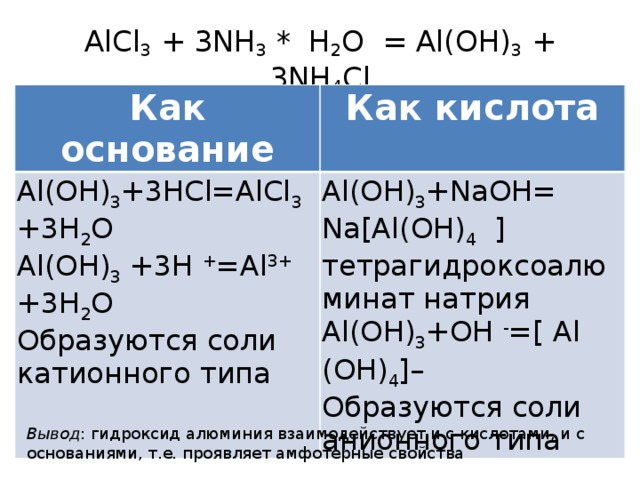
 Aluminium hydroxide, Al (OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic …
Aluminium hydroxide, Al (OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic …
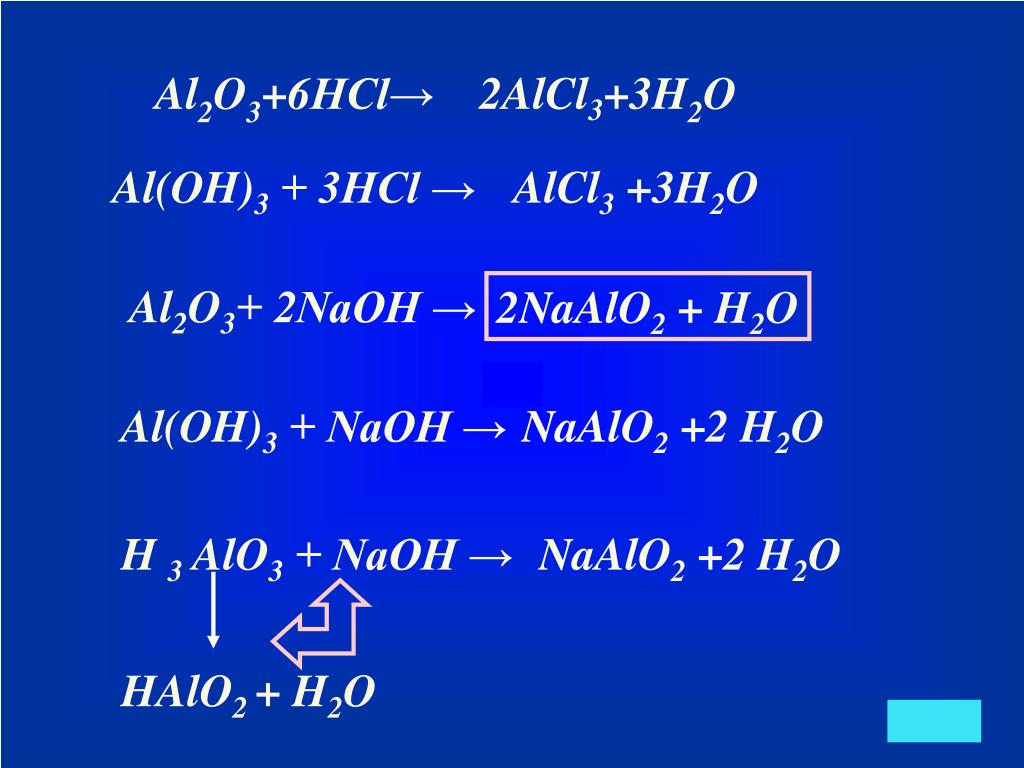
 Решенное и коэффициентами уравнение реакции Al2O3 + 3 H2O → 2 Al (OH)3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции Al2O3 + 3 H2O → 2 Al (OH)3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
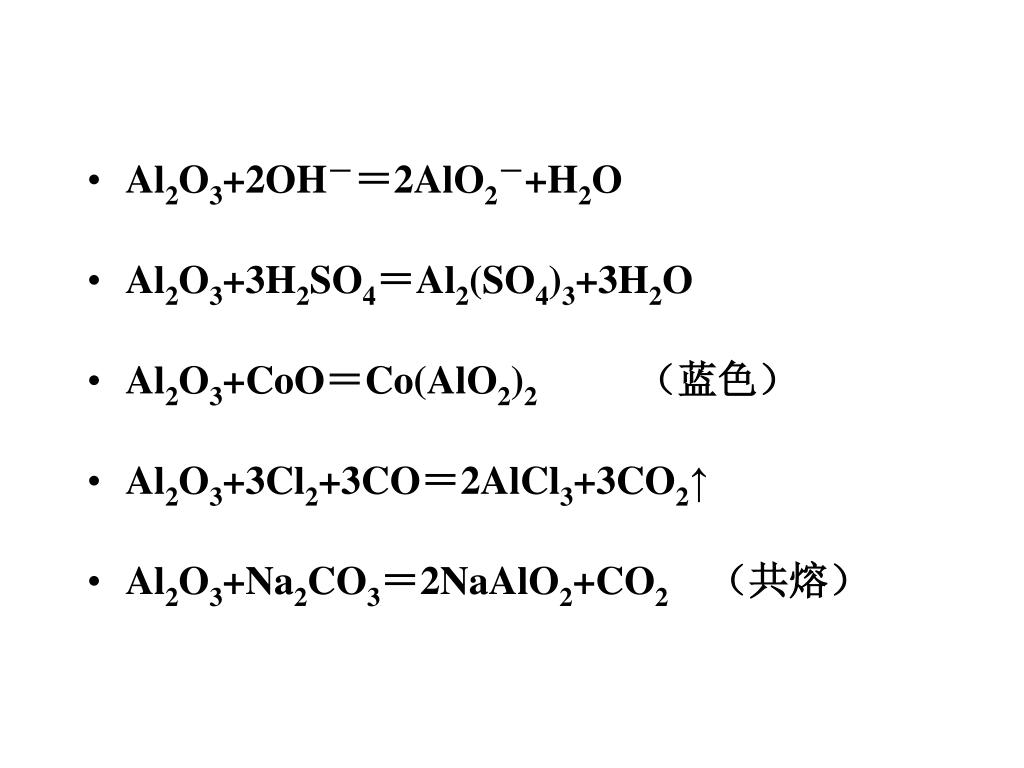 Balanced Chemical Equation – Solution. 2Al + 6H2O → 2Al (OH)3 + 3H2. The coefficients show the number of particles (atoms or molecules), and the indices show the …
Balanced Chemical Equation – Solution. 2Al + 6H2O → 2Al (OH)3 + 3H2. The coefficients show the number of particles (atoms or molecules), and the indices show the …
 A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
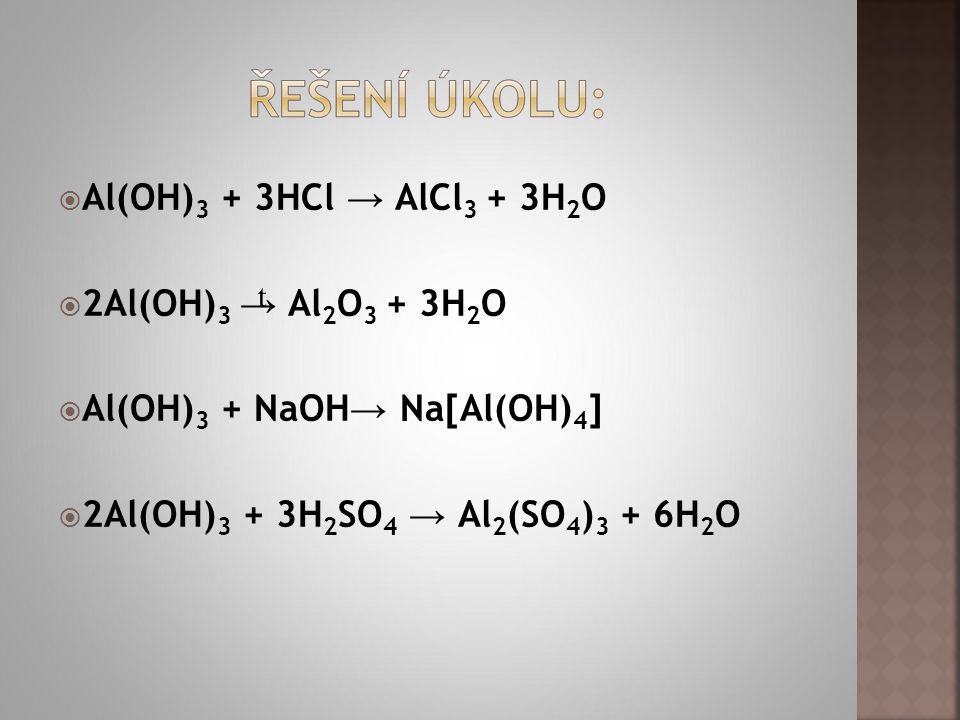
 Answer and Explanation: 1. The balanced equation is 2 A l (O H) 3 → A l 2 O 3 + 3 H 2 O. We need to add coefficients to the equation {eq}\rm Al (OH)_3 \to Al_2O_3 +.
Answer and Explanation: 1. The balanced equation is 2 A l (O H) 3 → A l 2 O 3 + 3 H 2 O. We need to add coefficients to the equation {eq}\rm Al (OH)_3 \to Al_2O_3 +.
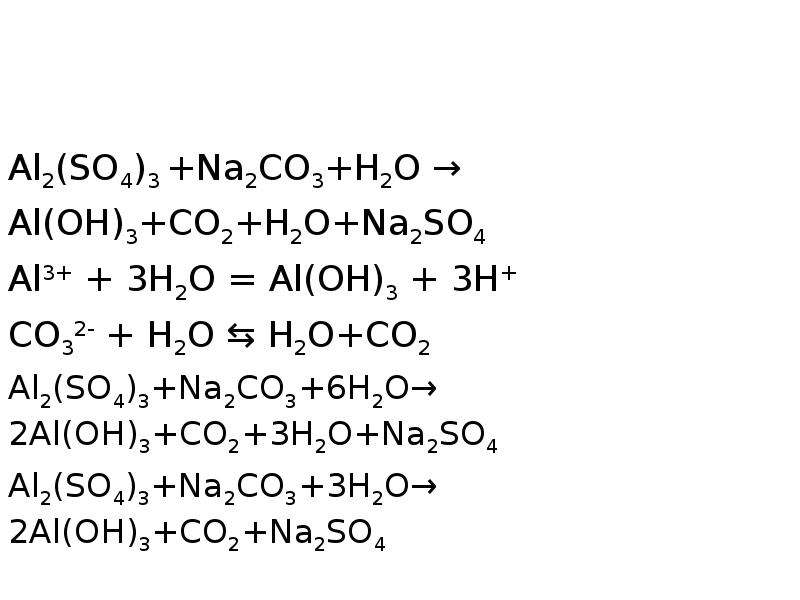
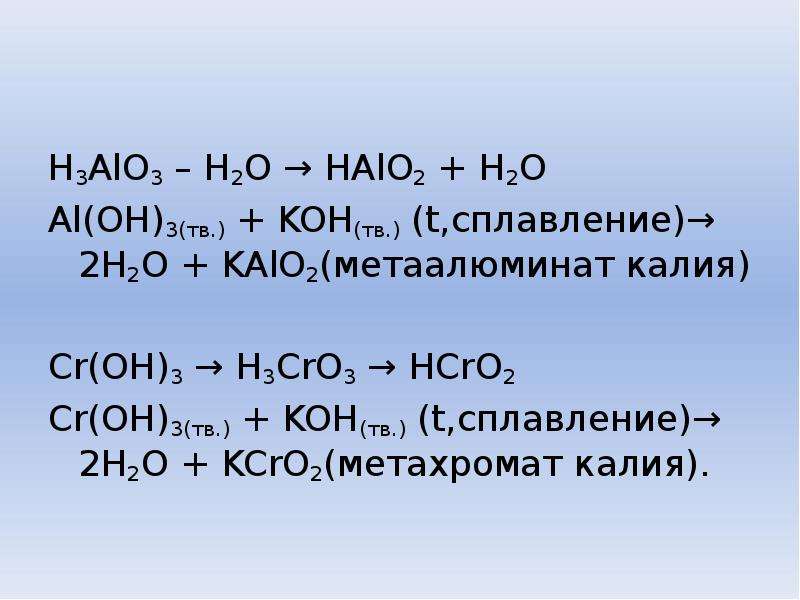 2Al(OH)3(s)+3H2SO4(aq)→Al2(SO4)3(aq)+6H2O(l) Это кислотно-щелочная реакция (нейтрализа́ция): Al(OH)3 является щелочным, H2SO4 представляет собой кислоту. …
2Al(OH)3(s)+3H2SO4(aq)→Al2(SO4)3(aq)+6H2O(l) Это кислотно-щелочная реакция (нейтрализа́ция): Al(OH)3 является щелочным, H2SO4 представляет собой кислоту. …
 1 H 2 O + 1 Al 2 O 3 = 1 Al(OH) 3 For each element, we check if the number of atoms is balanced on both sides of the equation. H is not balanced: 2 atoms in reagents and 3 atoms in products.
1 H 2 O + 1 Al 2 O 3 = 1 Al(OH) 3 For each element, we check if the number of atoms is balanced on both sides of the equation. H is not balanced: 2 atoms in reagents and 3 atoms in products.
Еще по теме:
![Al2O3 + 2 KOH + 3 H2O → 2 K[Al(OH)4] - Вычисленное … Foto 16](https://i3.wp.com/ru-static.z-dn.net/files/d9d/1fc939377cef9d2a32c07c70f94173f9.png?ssl=1)





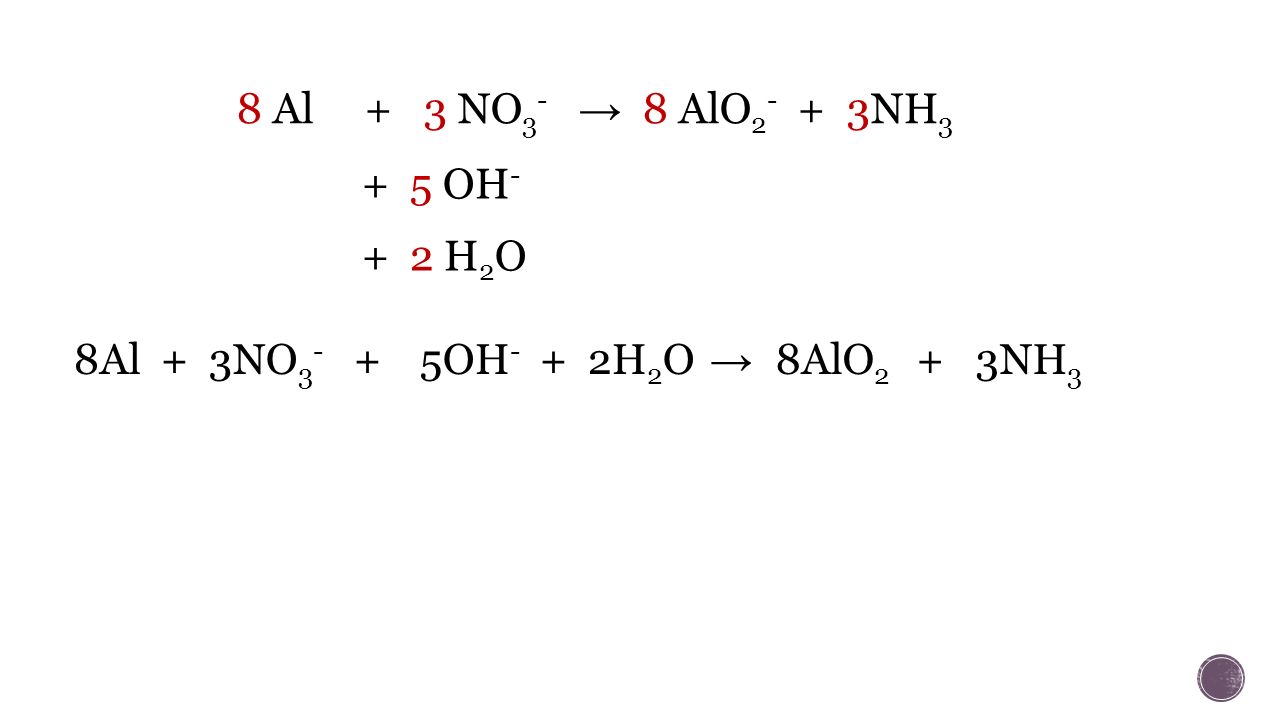






Еще по теме: