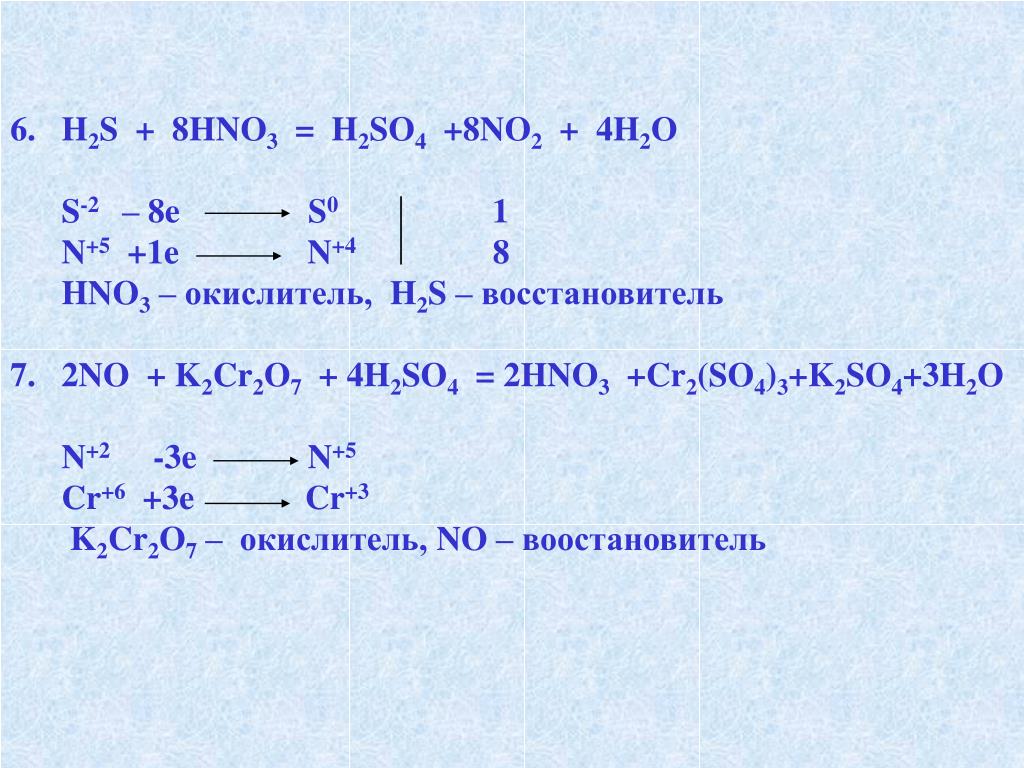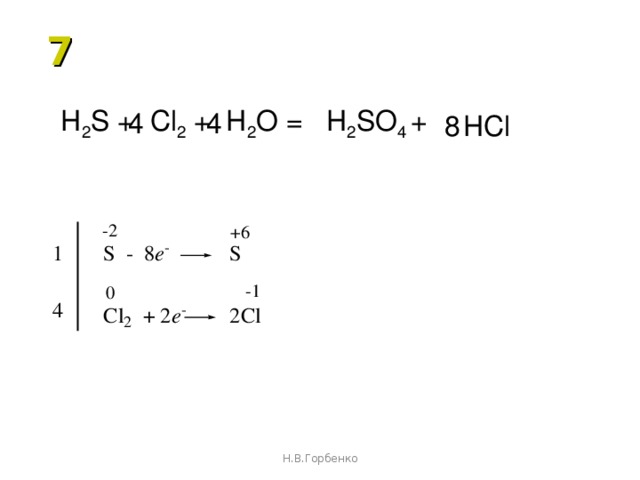 Решенное и коэффициентами уравнение реакции 2 h2s + so2 → 3 s + 2 h2o с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 h2s + so2 → 3 s + 2 h2o с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Reducing agent: In this reaction, H 2 S (Hydrogen sulphide) by gaining Oxygen atom, it is oxidized to H 2 O (Water) therefore, it acts as Reducing agent. Balance the following chemical …
Reducing agent: In this reaction, H 2 S (Hydrogen sulphide) by gaining Oxygen atom, it is oxidized to H 2 O (Water) therefore, it acts as Reducing agent. Balance the following chemical …
 Phản ứng SO 2 + H 2 S hay SO 2 tạo ra S thuộc loại phản ứng oxi hóa khử đã được cân bằng chính xác và chi tiết nhất. Bên cạnh đó là một số bài tập có liên quan về SO 2 có lời giải, mời …
Phản ứng SO 2 + H 2 S hay SO 2 tạo ra S thuộc loại phản ứng oxi hóa khử đã được cân bằng chính xác và chi tiết nhất. Bên cạnh đó là một số bài tập có liên quan về SO 2 có lời giải, mời …
 1 so 2 + 2 h 2 s = 1 s + 2 h 2 o S не сбалансирован: 3 атомов в реагентах и 1 атомов в продуктах. Чтобы сбалансировать S с обеих сторон, мы:
1 so 2 + 2 h 2 s = 1 s + 2 h 2 o S не сбалансирован: 3 атомов в реагентах и 1 атомов в продуктах. Чтобы сбалансировать S с обеих сторон, мы:
 Solved and balanced chemical equation SO2 + 2 H2S → 2 H2O + 3 S with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation SO2 + 2 H2S → 2 H2O + 3 S with completed products. Application for completing products and balancing equations.
 To balance the chemical equation, begin by multiplying the element or compound which has less number of atoms with a number that would make the number equal on both sides. Left hand …
To balance the chemical equation, begin by multiplying the element or compound which has less number of atoms with a number that would make the number equal on both sides. Left hand …
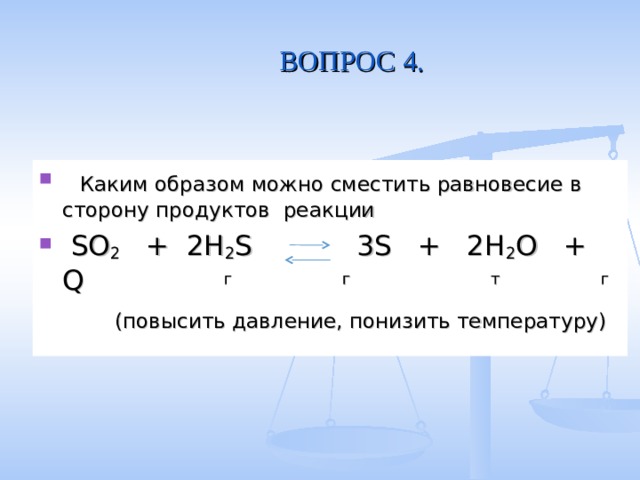
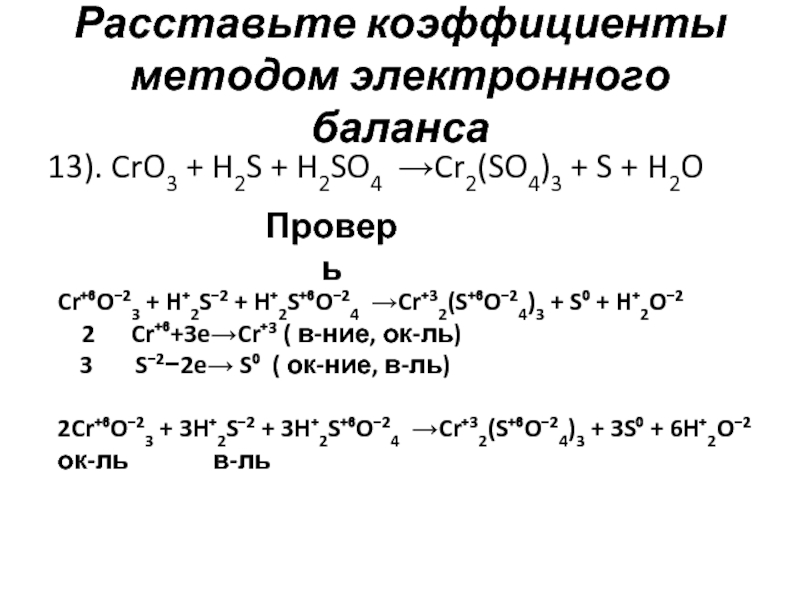
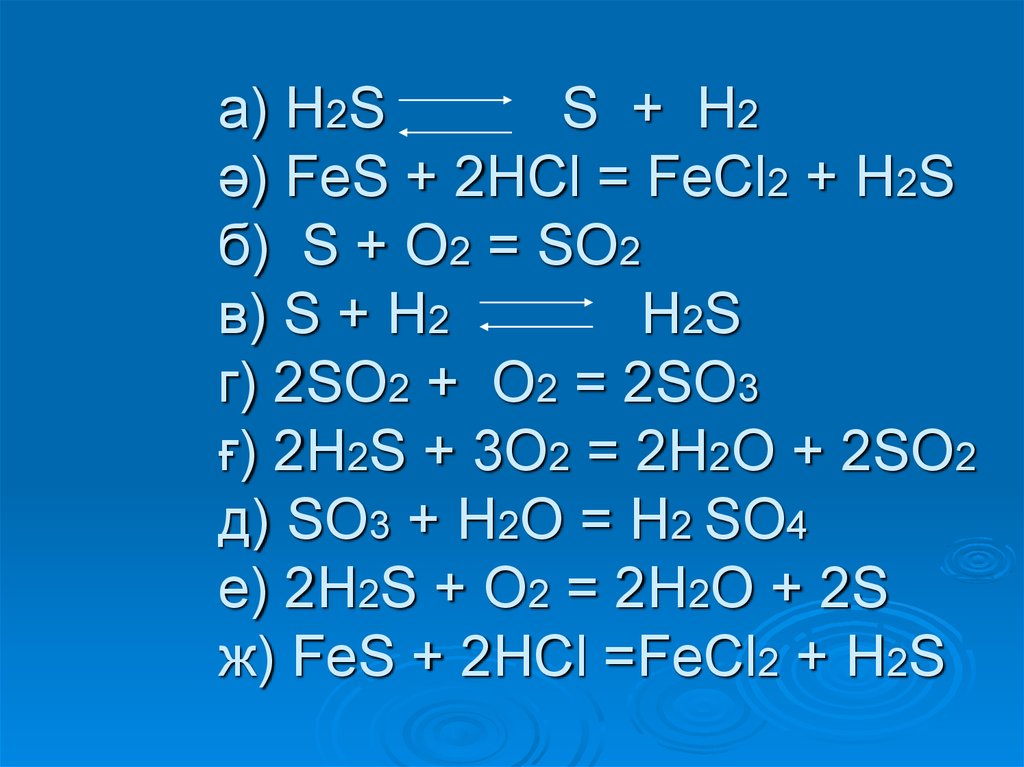 so2 + 2h2s = 3s + 2h2o; s(-2) - 2e = s(0)|2 восстановитель s(+4)+4e = s(0)|1 окислитель
so2 + 2h2s = 3s + 2h2o; s(-2) - 2e = s(0)|2 восстановитель s(+4)+4e = s(0)|1 окислитель
 1 SO 2 + 1 H 2 = 1 H 2 O + 1 H 2 S For each element, we check if the number of atoms is balanced on both sides of the equation. S is balanced: 1 atom in reagents and 1 atom in …
1 SO 2 + 1 H 2 = 1 H 2 O + 1 H 2 S For each element, we check if the number of atoms is balanced on both sides of the equation. S is balanced: 1 atom in reagents and 1 atom in …
 Решенное и коэффициентами уравнение реакции 2 H2S + SO2 → 2 H2O + 3 S с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 2 H2S + SO2 → 2 H2O + 3 S с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 H2S + SO2 = S8 + H2O is a Double Displacement (Metathesis) reaction where sixteen moles of Hydrogen Sulfide [H 2 S] and eight moles of Sulfur Dioxide [SO 2] react to form three moles of …
H2S + SO2 = S8 + H2O is a Double Displacement (Metathesis) reaction where sixteen moles of Hydrogen Sulfide [H 2 S] and eight moles of Sulfur Dioxide [SO 2] react to form three moles of …
 1 s + 2 h 2 o = 2 h 2 s + 1 so 2 S не сбалансирован: 1 атомов в реагентах и 3 атомов в продуктах. Чтобы сбалансировать S с обеих сторон, мы:
1 s + 2 h 2 o = 2 h 2 s + 1 so 2 S не сбалансирован: 1 атомов в реагентах и 3 атомов в продуктах. Чтобы сбалансировать S с обеих сторон, мы:
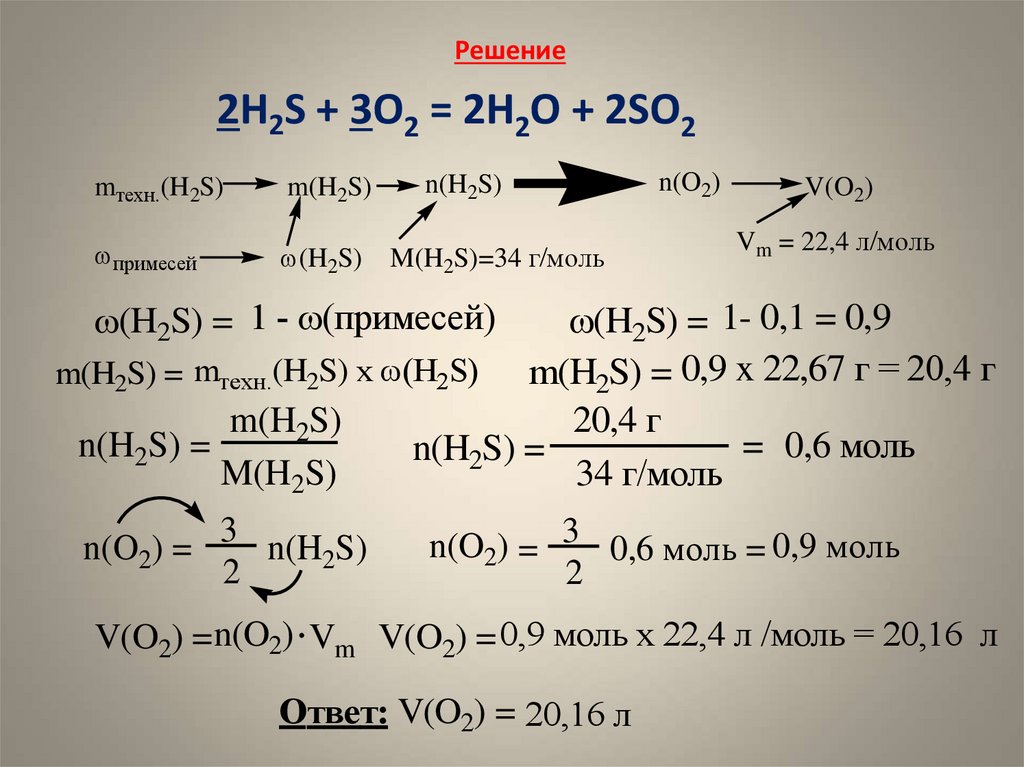
 Hydrogen sulfide reacts with sulfur dioxide to give H2O and S, H2S + SO2 = H2O + S (solid), unbalanced. If 6.0 L of H2S gas at 750 torr produced 3.2 g of sulfur, what is the …
Hydrogen sulfide reacts with sulfur dioxide to give H2O and S, H2S + SO2 = H2O + S (solid), unbalanced. If 6.0 L of H2S gas at 750 torr produced 3.2 g of sulfur, what is the …
 Solved and balanced chemical equation H2SO4 + H2S → SO2 + 2 H2O + S with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation H2SO4 + H2S → SO2 + 2 H2O + S with completed products. Application for completing products and balancing equations.
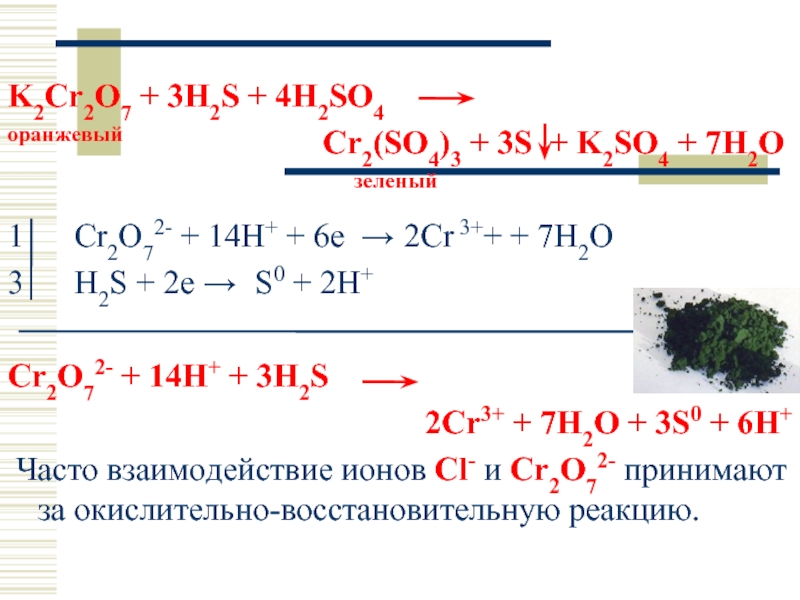
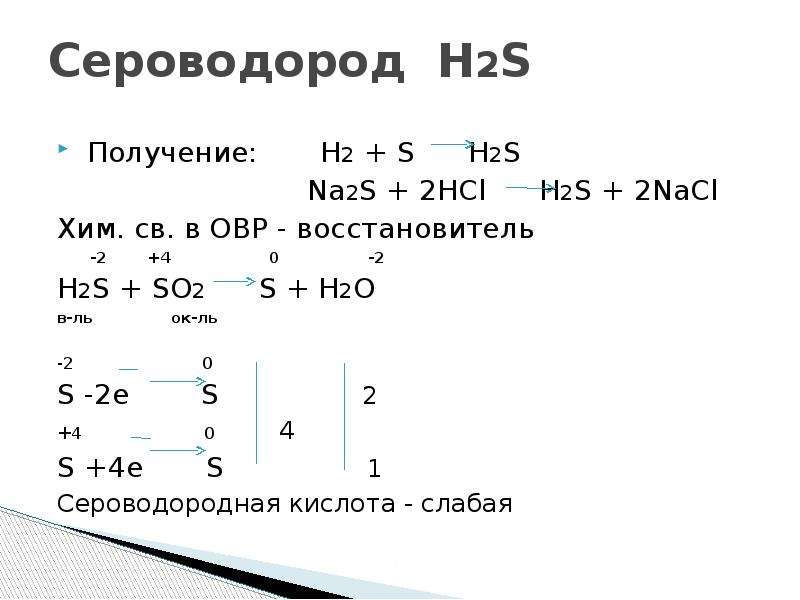
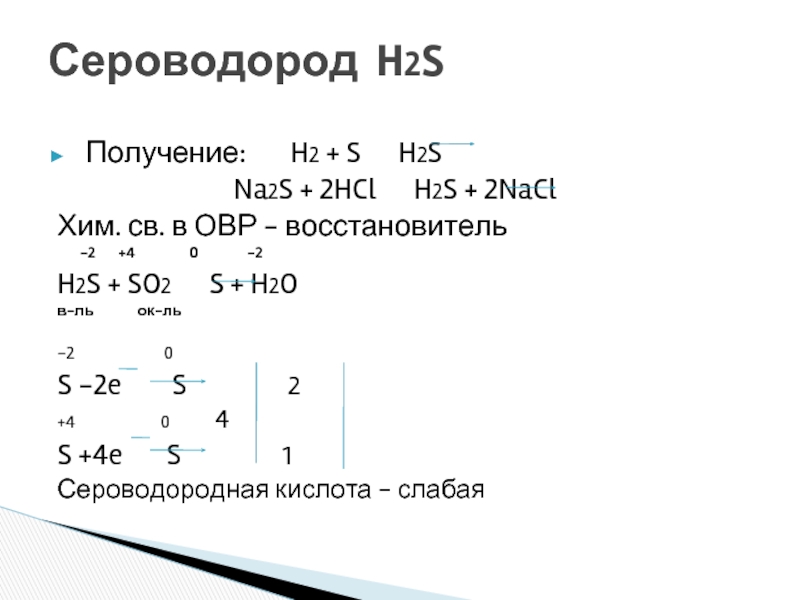
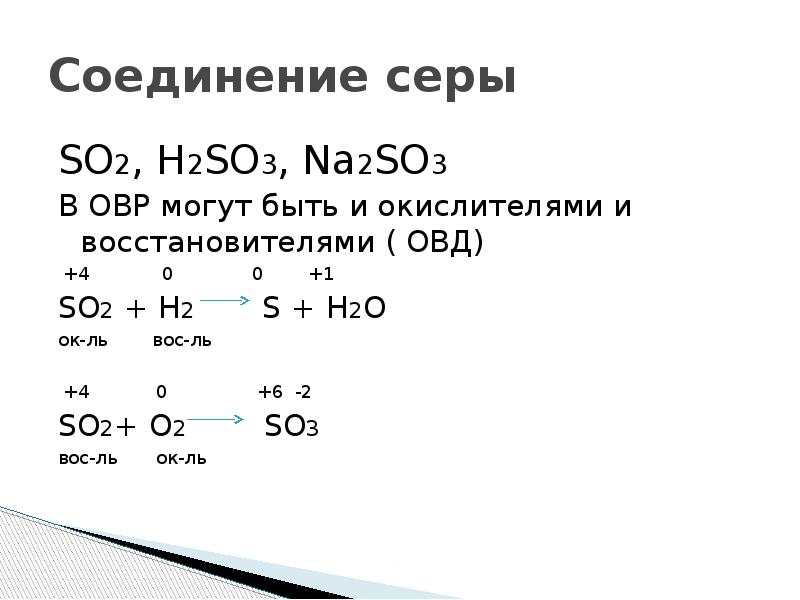
 SO 2 + H 2 S = S + H 2 O reaction This is a redox reaction (oxidation - reduction) . Sulphur atom in the sulphur dioxide molecule is reduced to sulfur while sulphur atom in the hydrogen …
SO 2 + H 2 S = S + H 2 O reaction This is a redox reaction (oxidation - reduction) . Sulphur atom in the sulphur dioxide molecule is reduced to sulfur while sulphur atom in the hydrogen …
 1 so 2 + 1 h 2 s = 1 h 2 o + 1 s For each element, we check if the number of atoms is balanced on both sides of the equation. O is not balanced: 2 atoms in reagents and 1 atom in products.
1 so 2 + 1 h 2 s = 1 h 2 o + 1 s For each element, we check if the number of atoms is balanced on both sides of the equation. O is not balanced: 2 atoms in reagents and 1 atom in products.
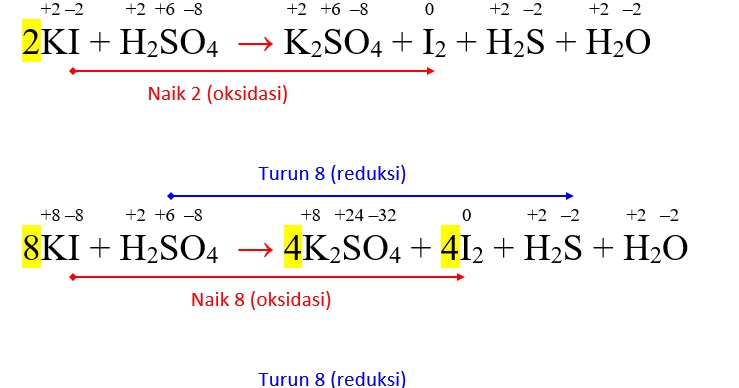
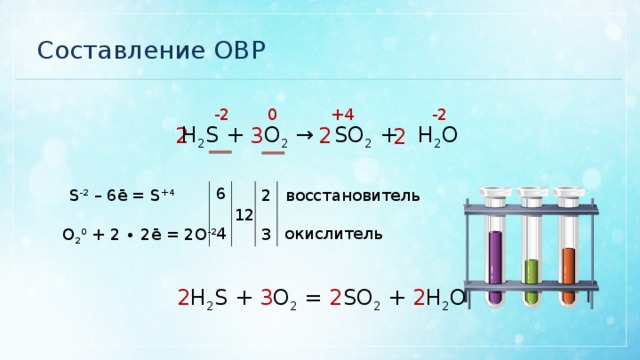
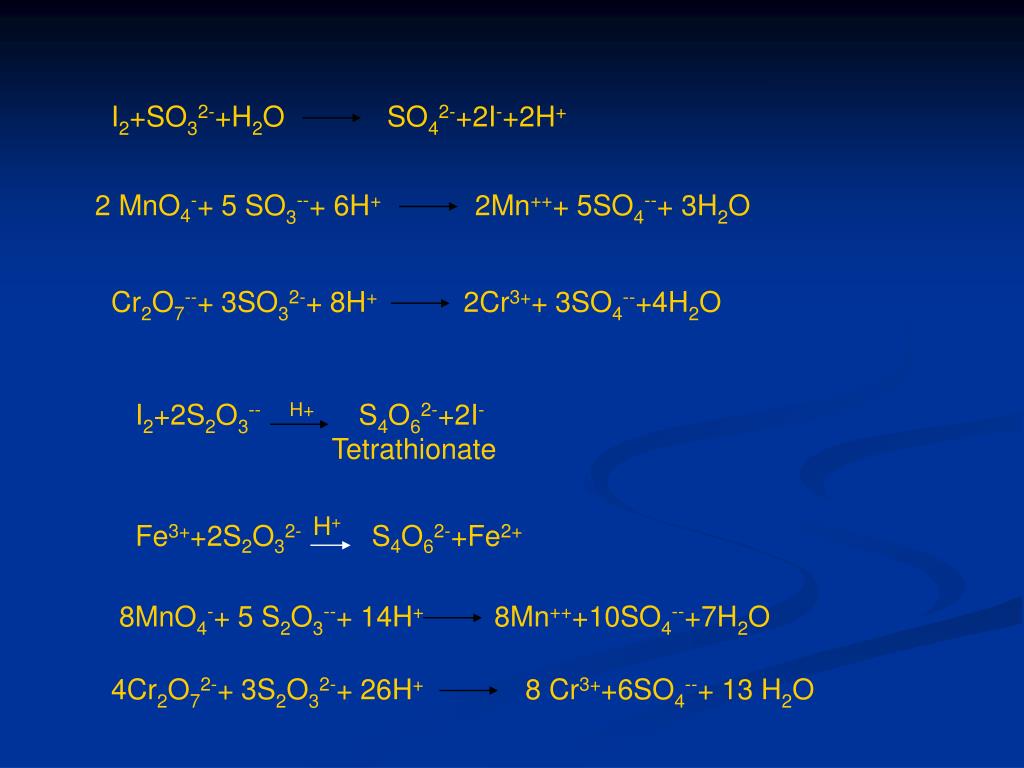
 В химической реакции, схема которой H2S + SO2 → S + H2O, изменение степени окисления восстановителя происходит по схеме Выберите один ответ: a. S+4 → S0 b. S0 → S-2 c. …
В химической реакции, схема которой H2S + SO2 → S + H2O, изменение степени окисления восстановителя происходит по схеме Выберите один ответ: a. S+4 → S0 b. S0 → S-2 c. …
Еще по теме:


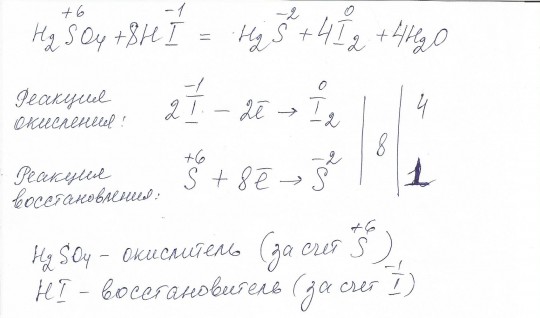








Еще по теме: