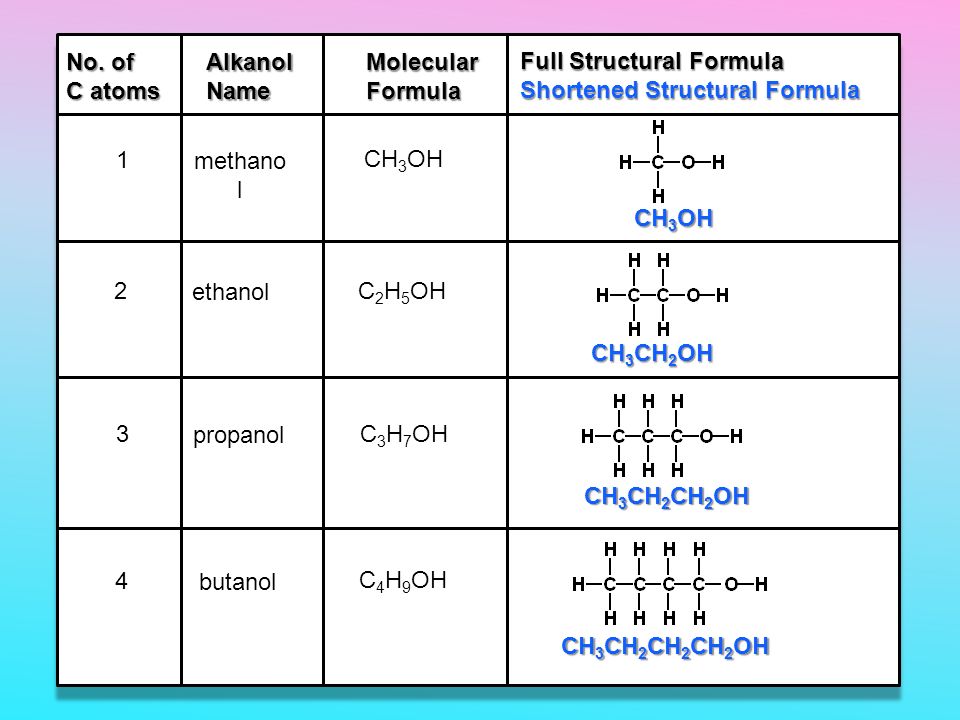
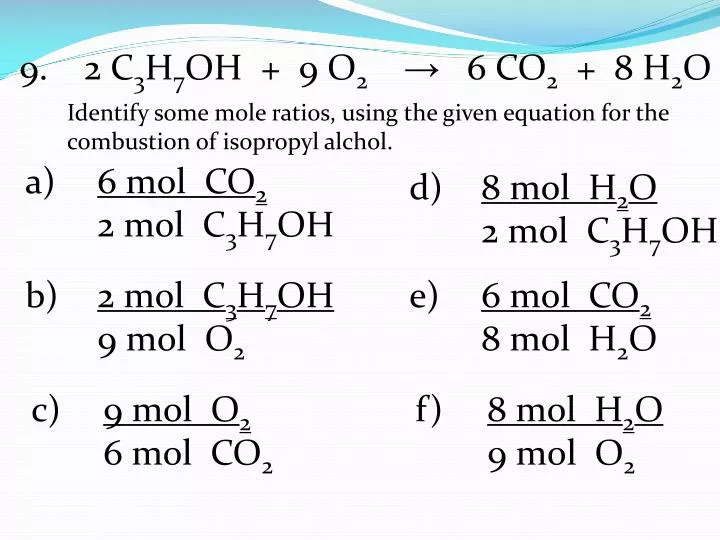
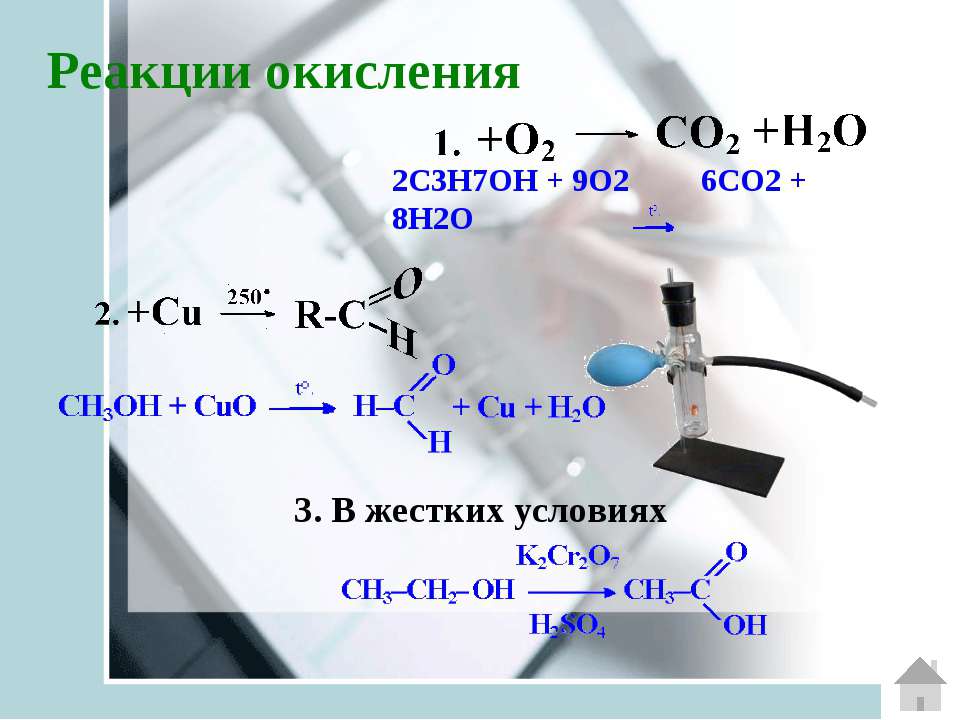
 C3H7OH + O2 = CO2 + H2O is a Combustion reaction where two moles of Isopropyl Alcohol [C 3 H 7 OH] and nine moles of Dioxygen [O 2] react to form six moles of Carbon Dioxide [CO 2] and eight moles of Water [H 2 O]
C3H7OH + O2 = CO2 + H2O is a Combustion reaction where two moles of Isopropyl Alcohol [C 3 H 7 OH] and nine moles of Dioxygen [O 2] react to form six moles of Carbon Dioxide [CO 2] and eight moles of Water [H 2 O]
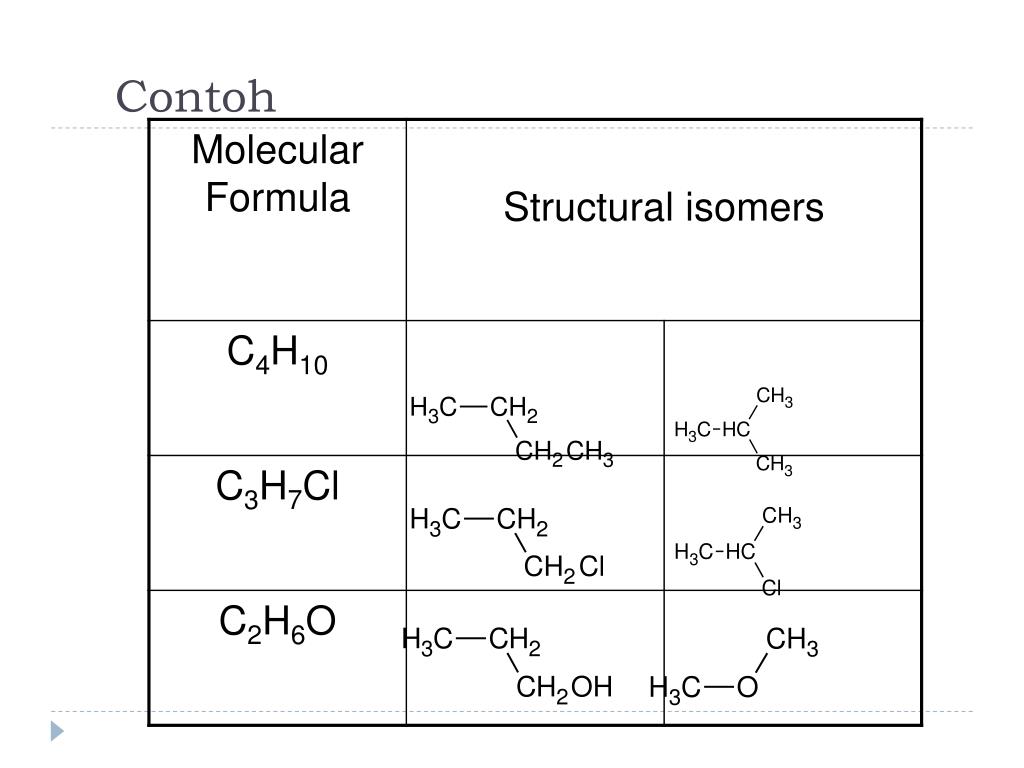
 Решенное и коэффициентами уравнение реакции C3H7OH + O2 → CH3CH2COOH + H2O с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции C3H7OH + O2 → CH3CH2COOH + H2O с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Enter an equation of a chemical reaction and click 'Balance'. The answer will appear below. Always use the upper case for the first character in the element name and the lower case for …
Enter an equation of a chemical reaction and click 'Balance'. The answer will appear below. Always use the upper case for the first character in the element name and the lower case for …
 Solved and balanced chemical equation C3H7OH + O2 → CH3CH2COOH + H2O with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation C3H7OH + O2 → CH3CH2COOH + H2O with completed products. Application for completing products and balancing equations.
 To balance C3H7OH + O2 = CO2 + H2O you'll need to be sure to count all of atoms on each side of the chemical equation..more. In this video we'll balance the equation …
To balance C3H7OH + O2 = CO2 + H2O you'll need to be sure to count all of atoms on each side of the chemical equation..more. In this video we'll balance the equation …
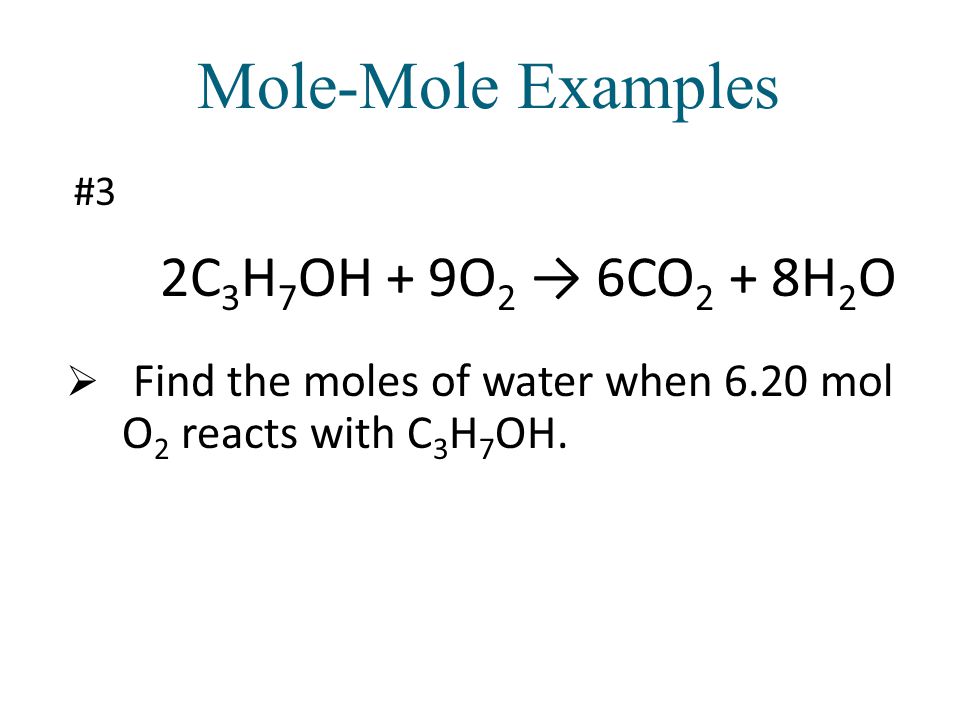
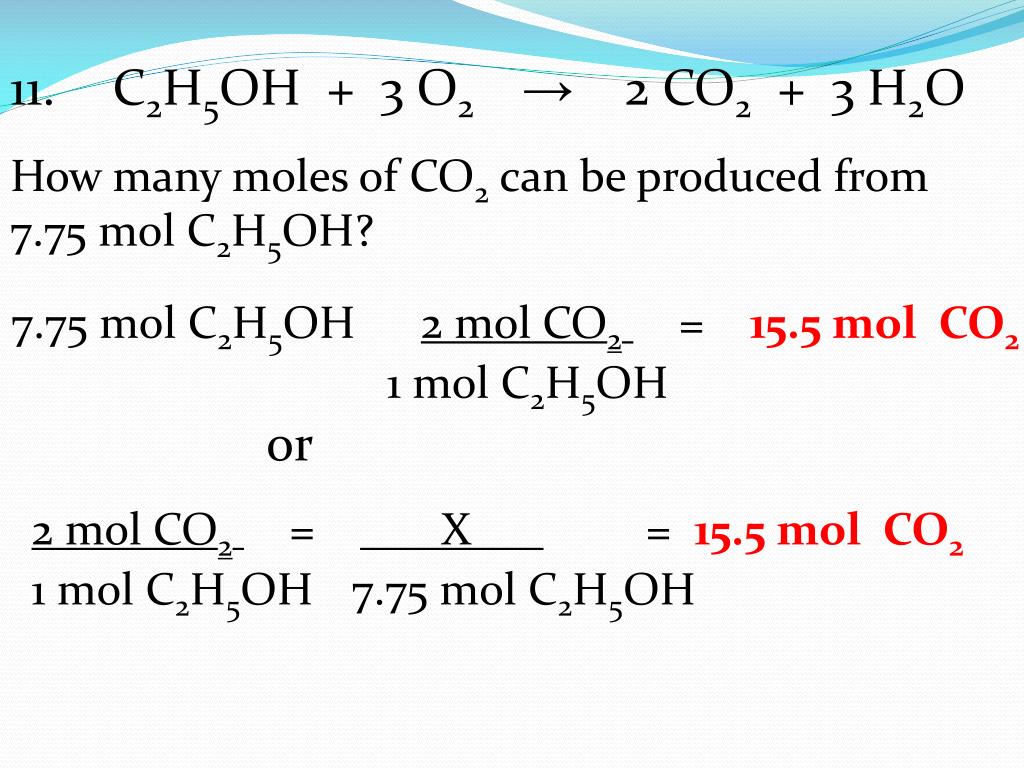
 The stoichiometry calculator above shows the mole ratios/coefficients of the balanced equation, 2C3H7OH + 9O2 = 6CO2 + 8H2O. Enter the amount of any of the substances to determine …
The stoichiometry calculator above shows the mole ratios/coefficients of the balanced equation, 2C3H7OH + 9O2 = 6CO2 + 8H2O. Enter the amount of any of the substances to determine …
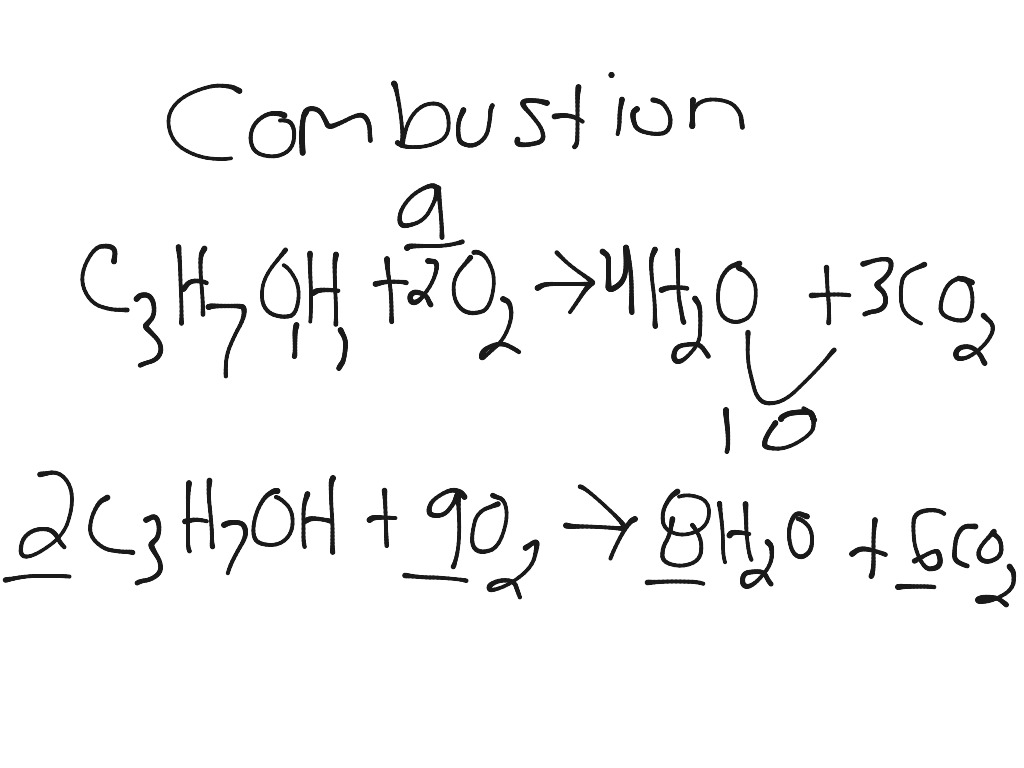 O2 + C3H7OH = CO2 + H2O is a Combustion reaction where nine moles of Dioxygen [O 2] and two moles of Isopropyl Alcohol [C 3 H 7 OH] react to form six moles of Carbon Dioxide [CO 2] …
O2 + C3H7OH = CO2 + H2O is a Combustion reaction where nine moles of Dioxygen [O 2] and two moles of Isopropyl Alcohol [C 3 H 7 OH] react to form six moles of Carbon Dioxide [CO 2] …
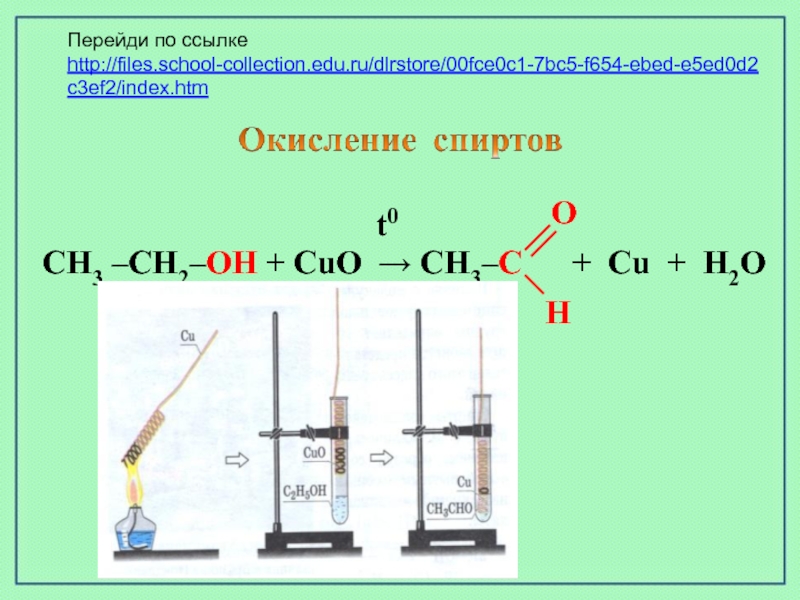
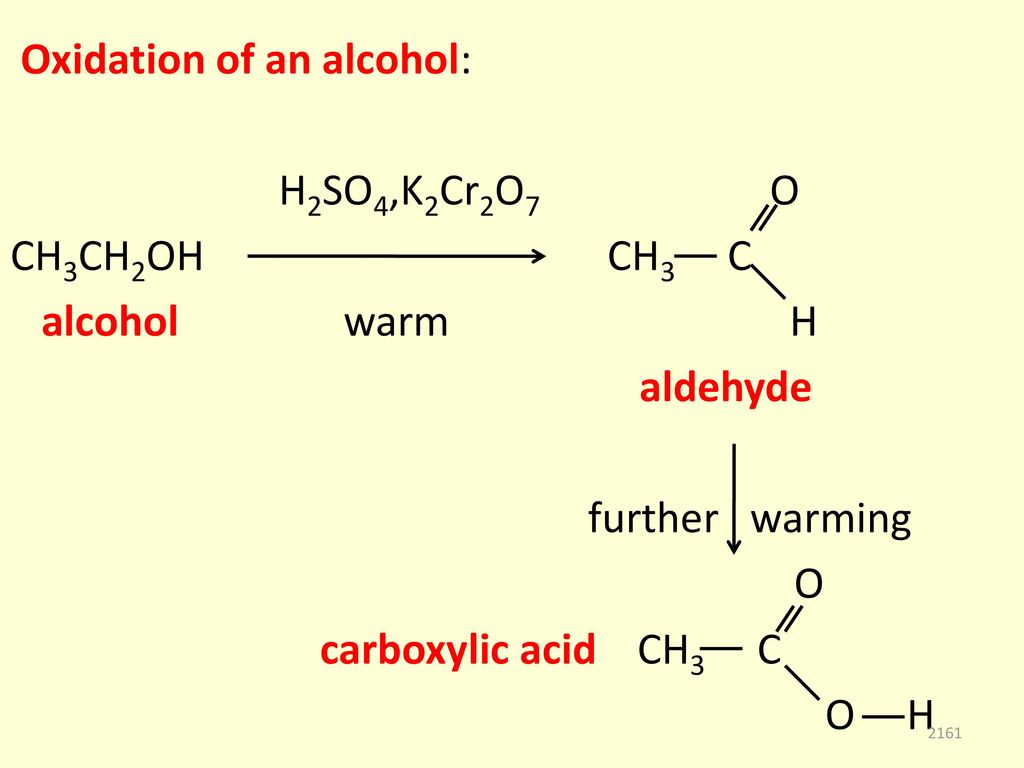
 Alcohols react with oxygen in a similar way to hydrocarbons, where they produce carbon dioxide (CO2) and water (H 2O). Therefore, the reaction of the complete combustion of …
Alcohols react with oxygen in a similar way to hydrocarbons, where they produce carbon dioxide (CO2) and water (H 2O). Therefore, the reaction of the complete combustion of …
 Закінчіть рівняння реакції, вкажіть суму коефіцієнтів. C3H7OH + O2 → CO2 + H2O. Відповіді на всі питання - на одному сайті! Застрягли на складному завданні з курсу старших …
Закінчіть рівняння реакції, вкажіть суму коефіцієнтів. C3H7OH + O2 → CO2 + H2O. Відповіді на всі питання - на одному сайті! Застрягли на складному завданні з курсу старших …
 Balance the following reaction: C 3 H 7 O H + O 2 → C O 2 + H 2 O. The given chemical reaction is a combustion reaction since it involves the oxygen molecule as the reactant and leads to.
Balance the following reaction: C 3 H 7 O H + O 2 → C O 2 + H 2 O. The given chemical reaction is a combustion reaction since it involves the oxygen molecule as the reactant and leads to.
 C3H7OH + O2 CO2 + H2O. Balance the following reaction: CH4 + O2 CO2 + H2O. In that equation above, 31.2 kJ of energy are released for every gram of methane burned. How much …
C3H7OH + O2 CO2 + H2O. Balance the following reaction: CH4 + O2 CO2 + H2O. In that equation above, 31.2 kJ of energy are released for every gram of methane burned. How much …
 Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
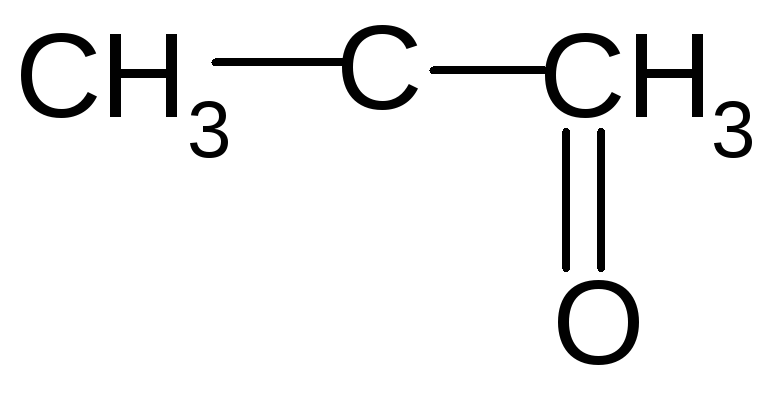
 Both 1-Propanol (C3H7OH) and 2-propanol react with oxygen (O2) to make carbon dioxide (CO2) and water (H2O). Complete combustion does NOT give carbon monoxid.
Both 1-Propanol (C3H7OH) and 2-propanol react with oxygen (O2) to make carbon dioxide (CO2) and water (H2O). Complete combustion does NOT give carbon monoxid.
 For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but PhC2H5 + O2 = PhOH + CO2 + H2O will Compound states [like (s) (aq) or (g)] are not required.
For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but PhC2H5 + O2 = PhOH + CO2 + H2O will Compound states [like (s) (aq) or (g)] are not required.
 Solved and balanced chemical equation O2 + C3H7OH → H2O + CH3CH2COOH with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation O2 + C3H7OH → H2O + CH3CH2COOH with completed products. Application for completing products and balancing equations.
Еще по теме:








Еще по теме: