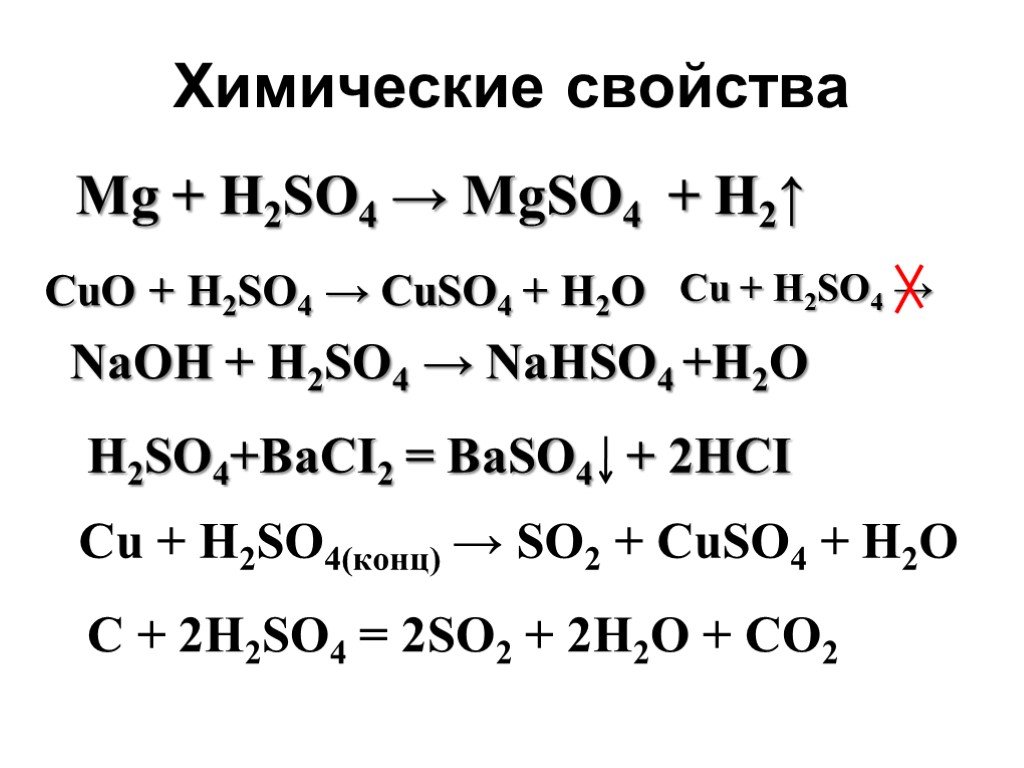
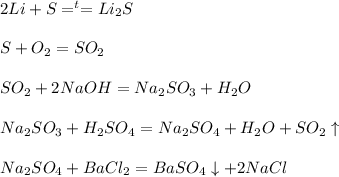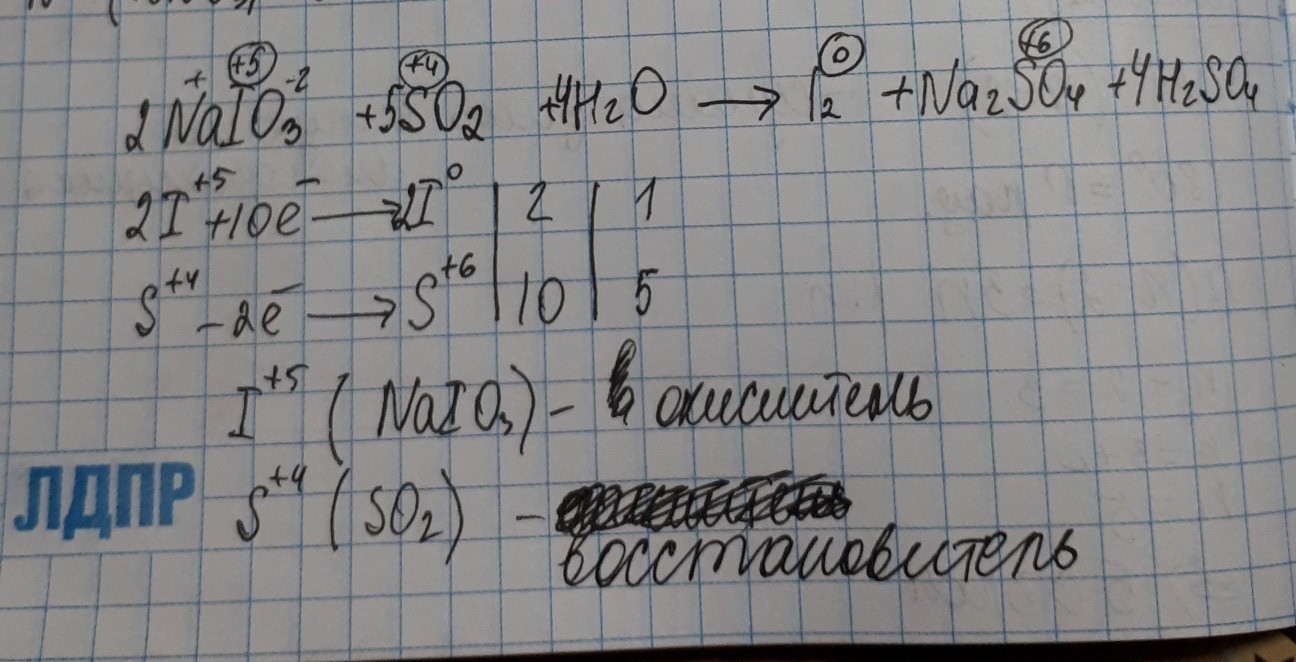 Решенное и коэффициентами уравнение реакции naoh + h2so4 → nahso4 + h2o с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции naoh + h2so4 → nahso4 + h2o с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 Solution. Chemical equations are a simple method to illustrate any chemical reaction taking place. It involves the chemical formula of each reactant and product. The reactants and products are …
Solution. Chemical equations are a simple method to illustrate any chemical reaction taking place. It involves the chemical formula of each reactant and product. The reactants and products are …
 NaOH + H2SO4 = Na2SO4 + H2O is a Double Displacement (Metathesis) reaction where two moles of aqueous Sodium Hydroxide [NaOH] and one mole of aqueous Sulfuric Acid [H 2 SO …
NaOH + H2SO4 = Na2SO4 + H2O is a Double Displacement (Metathesis) reaction where two moles of aqueous Sodium Hydroxide [NaOH] and one mole of aqueous Sulfuric Acid [H 2 SO …
 1 H 2 SO 4 (aq) + 1 NaOH = 1 Na 2 SO 4 (s) + 1 H 2 O For each element, we check if the number of atoms is balanced on both sides of the equation. S is balanced: 1 atom in reagents and 1 …
1 H 2 SO 4 (aq) + 1 NaOH = 1 Na 2 SO 4 (s) + 1 H 2 O For each element, we check if the number of atoms is balanced on both sides of the equation. S is balanced: 1 atom in reagents and 1 …
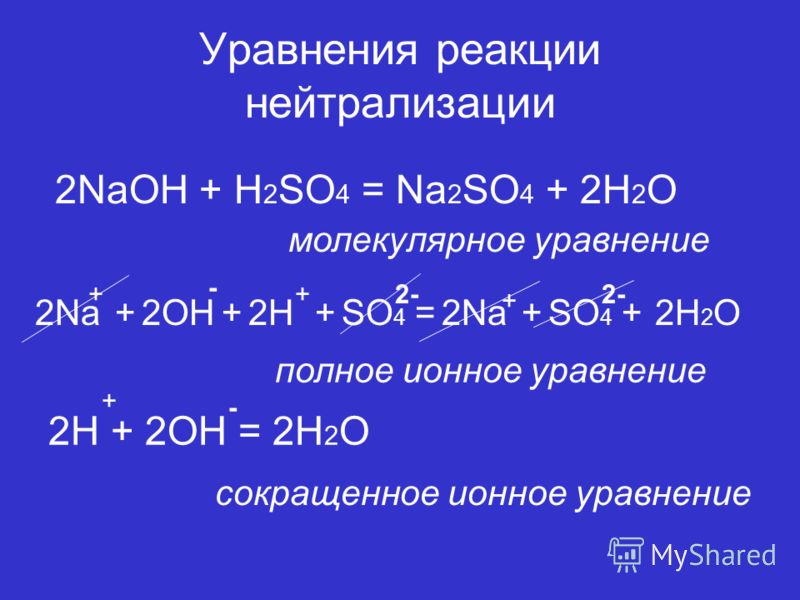
 Чистое ионное уравнение. 2 H {+} + SO 4{-2} + Na {+} + OH {-} = H 2 OH + NaSO 4. Балансировка шаг за шагом методом проверки. Давайте сбалансируем это уравнение, …
Чистое ионное уравнение. 2 H {+} + SO 4{-2} + Na {+} + OH {-} = H 2 OH + NaSO 4. Балансировка шаг за шагом методом проверки. Давайте сбалансируем это уравнение, …
 Balanced Chemical Equation – Solution. H2SO4 + 2NaOH → Na2SO4 + 2H2O. The coefficients show the number of particles (atoms or molecules), and the indices show the …
Balanced Chemical Equation – Solution. H2SO4 + 2NaOH → Na2SO4 + 2H2O. The coefficients show the number of particles (atoms or molecules), and the indices show the …
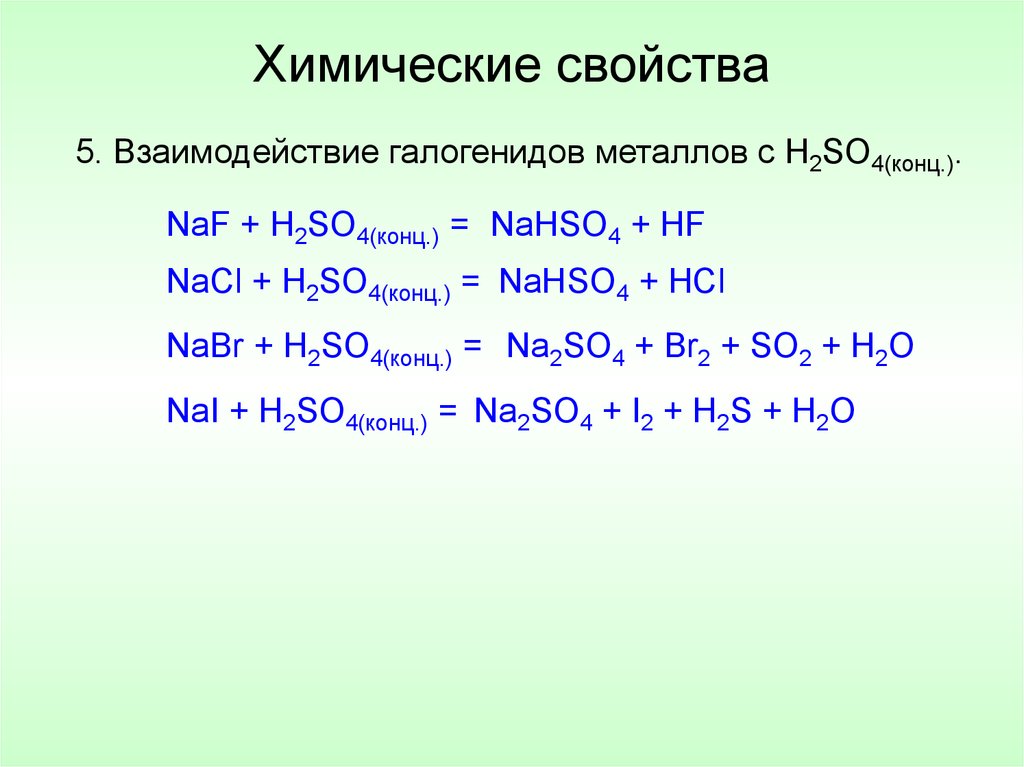
 NaOH + H2SO4 = H2O + Na2SO4 - Сбалансированное химическое уравнение, лимитирующий реагент и стехиометрия. Балансировка химического уравнения - онлайн …
NaOH + H2SO4 = H2O + Na2SO4 - Сбалансированное химическое уравнение, лимитирующий реагент и стехиометрия. Балансировка химического уравнения - онлайн …
 Answer. NaOH + H 2 SO 4 → Na 2 SO 4 + H 2 O. To balance Sodium, Multiply NaOH by 2. 2 NaOH + H 2 SO 4 → Na 2 SO 4 + H 2 O. To balance Hydrogen, Multiply H 2 O …
Answer. NaOH + H 2 SO 4 → Na 2 SO 4 + H 2 O. To balance Sodium, Multiply NaOH by 2. 2 NaOH + H 2 SO 4 → Na 2 SO 4 + H 2 O. To balance Hydrogen, Multiply H 2 O …
 It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of hydrogen (H₂) with oxygen (O₂) to form water (H₂O), …
It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of hydrogen (H₂) with oxygen (O₂) to form water (H₂O), …
 A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of …
 Solution. Balancing of chemical equation: Step 1: Write unbalanced equation. NaOH + H SO 2 4 → Na SO 2 4 + H O 2. Step 2: Write down the number of atoms of each element present on …
Solution. Balancing of chemical equation: Step 1: Write unbalanced equation. NaOH + H SO 2 4 → Na SO 2 4 + H O 2. Step 2: Write down the number of atoms of each element present on …
 This article is about the naoh + h2so4 (Reaction mixture) and explains how a reaction occurs, product formation, balancing, and how to proceed with titration of given …
This article is about the naoh + h2so4 (Reaction mixture) and explains how a reaction occurs, product formation, balancing, and how to proceed with titration of given …
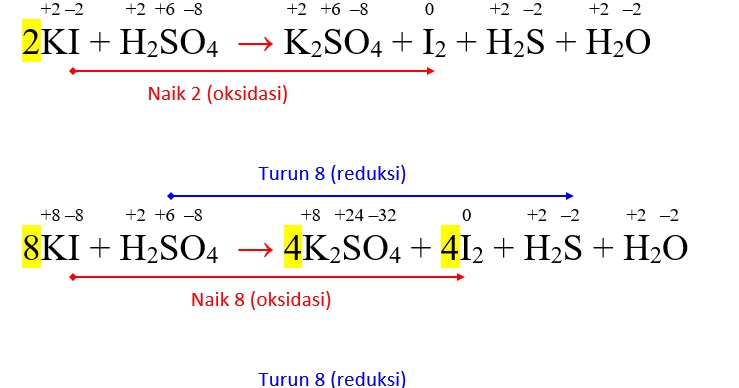
 1 H 2 SO 4 + 2 NaOH = 1 Na 2 SO 4 + 2 H 2 O O сбалансирован: 6 атомов в реагентах и 6 атомов в продуктах. All atoms are now balanced and the whole equation is fully balanced: H …
1 H 2 SO 4 + 2 NaOH = 1 Na 2 SO 4 + 2 H 2 O O сбалансирован: 6 атомов в реагентах и 6 атомов в продуктах. All atoms are now balanced and the whole equation is fully balanced: H …
 Go Pro Now. H2SO4+NaOH -> Na2SO4+H2O. Natural Language. Math Input. Extended Keyboard. Upload. Have a question about using Wolfram|Alpha? Compute answers using …
Go Pro Now. H2SO4+NaOH -> Na2SO4+H2O. Natural Language. Math Input. Extended Keyboard. Upload. Have a question about using Wolfram|Alpha? Compute answers using …
 Решенное и коэффициентами уравнение реакции NaHSO4 + H2O → NaOH + H2SO4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции NaHSO4 + H2O → NaOH + H2SO4 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
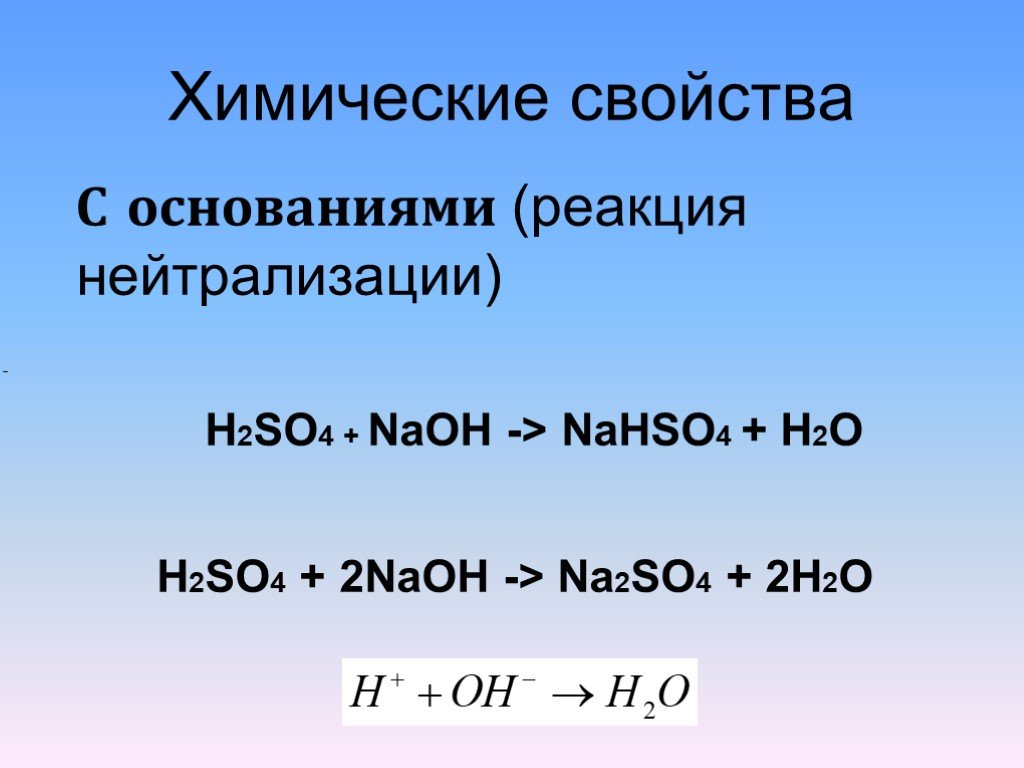
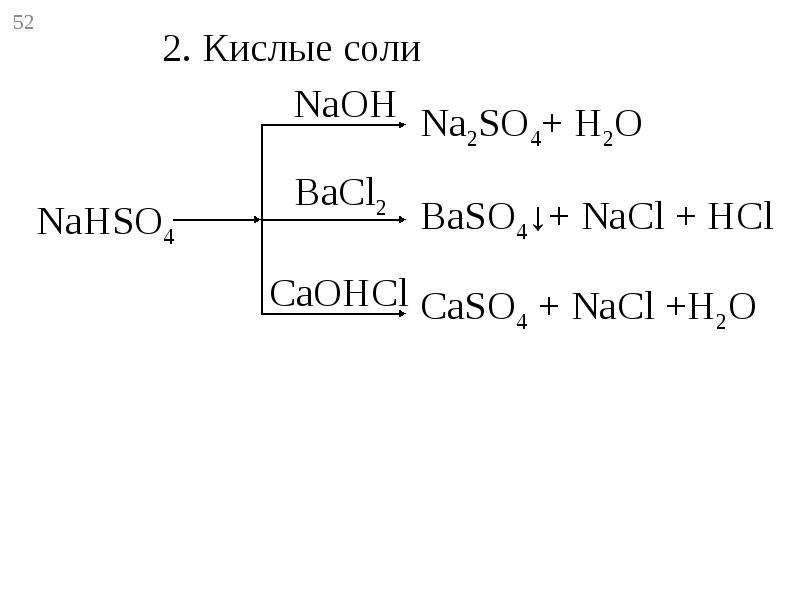
 The question states that when titrating H2SO4 against NaOH, both Na2SO4 (H2SO4+ 2NaOH—> Na2SO4 + 2H2O) and NaHSO4 (H2SO4+ NaOH —> NaHSO4 + H2O) …
The question states that when titrating H2SO4 against NaOH, both Na2SO4 (H2SO4+ 2NaOH—> Na2SO4 + 2H2O) and NaHSO4 (H2SO4+ NaOH —> NaHSO4 + H2O) …
Еще по теме:










Еще по теме: